Measurement of surface and interfacial tension- Capillary rise method and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Capillary Rise Method: The capillary rise method is considered to be the most accurate way of measuring surface tension as the surface of the liquid is undisturbed during the measurement. However, the method is not suitable for measuring the interfacial tension.
If a capillary tube is immersed in a liquid, provided the angle of contact that the liquid makes with the capillary tube is less than 90°, the liquid will rise in the tube to a certain height.
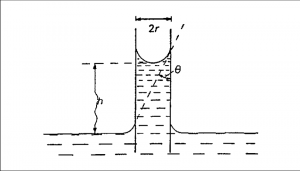
Fig 1 – Capillary rise method(taken from physic chemical basis of surface phenomena slideplayer)
The liquid rises in the capillary because the adhesion force between the water molecules and capillary wall is greater than the cohesion force between the water molecules. The liquid continues to rise in the capillary till the upward movement due to surface tension is just balanced by the downward force of gravity due to the weight of the liquid. By measuring the rise in the capillary, it is possible to determine the surface tension of the liquid.
Upward force due to surface tension
If the tube is small in diameter, the meniscus can be considered to be hemispherical, and the radius of curvature will be
rcosⱷ
The total upward force along the inside circumference of the capillary is given by
F = ᵞ2πr cosⱷ
where ⱷ is the contact angle between the surface of the liquid and the capillary wall and r the inside radius of the capillary tube. When a liquid such as water wets the surface of the capillary tube, ⱷ is taken as unity. Then, the upward force is given by
F = ᵞ2πr
Downward force due to the weight of the liquid
The downward force due to the weight of the liquid column is given by
W = mg = VǷg = πr2hǷg
where πr2
is the cross-sectional area of the capillary tube, h the height to which the liquid rises in the capillary, U the density of the liquid and g the acceleration due to gravity.
Equating the two forces at equilibrium, we get:
ᵞ2πr = πr2hǷg
ᵞ = r hǷg / 2
To determine the surface tension of a liquid, a capillary tube of known diameter is dipped into the same liquid contained in a vessel. The height to which the liquid rises in the capillary is determined with the help of a travelling microscope. The density of the liquid is determined using a pycnometer. The values obtained are then substituted in the equation to obtain the surface tension.
- Capillary tube should have a uniform diameter throughout its length.
- Outer vessels must have a larger diameter compared with the capillary.
Multiple choice questions (MCQs)
1.The capillary rise method is considered to be the most accurate way of measuring
a)surface tension
b)interfacial tension
c)both of these
d)none of these
2.What is the main result of adding surfactants into a liquid composed of two immiscible phases such as oil and water?
a)Reduction in the interfacial tension between the phases
b)Increase in the interfacial tension between the phases
c)Catalysation of a chemical reaction between the phases
d)Nothing happens
3.At what angle the liquid will rise in the tube to a certain height?
a)45 degree
b)90 degree
c)120 degree
d)360 degree
4.Why water rises in the capillary?
a)because the adhesion force between the water molecules and capillary wall is greater than the cohesion force between the water molecules
b)because the adhesion force between the water molecules and capillary wall is lesser than the cohesion force between the water molecules
c)because the adhesion force between the water molecules and capillary wall is to the cohesion force between the water molecules
d)None of these
5.The liquid continues to rise in the capillary till the upward movement due to
a)surface tension is just balanced by the downward force of gravity due to the weight of the liquid
b)surface tension is just balanced by the upward force of walls of capillary
c)both of these
d)none of these
6.If the tube is small in diameter, the meniscus can be considered to be
a)spherical
b)hemispherical
c)flat
d)circular
7.The total upward force along the inside circumference of the capillary is given by F = ᵞ2πr cosⱷ, where ⱷ is
a)contact angle between the surface of the liquid and the capillary wall
b)contact angle between the surface of the liquid and the base of capillary
c)contact angle between the surface of the liquid and gravitational force
d)All of the above
8.Which of the following apparatus can be used to determine surface tension
a)Rheometer
b)Stalagmometer
c)Du Nouy tensiometer
d)Ostwald viscometer
9.The downward force due to the weight of the liquid column is given by
a)W = mg = VǷg = πrhǷg
b)W = m = VǷg = πr2hǷg
c)W = mg = VǷg = πr2hǷg
d)W = mg = VǷg = πr3hǷg
10.Interfacial tension is applied on
a)Miscible liquids
b)Immiscible liquids
c)Both
d)None of the above
11.The density of the liquid is determined using a
a)Rheometer
b)Stalagmometer
c)Du Nouy tensiometer
d)pycnometer
12.Adhesive force>cohesive force, then what occurs
a)Wetting
b)Spreading
c)Capillary rising
d)All of these
13.Rise of liquid in capillary tube does not depends upon on:
a)Angle of contact
b)Density of liquid
c)Radius of capillary tube
d)Atmospheric pressure
14.Kerosene in the wick of lantern arises up because of
a)Surface tension of oil
b)Dilution of oil through wick
c)Negligible viscosity
d)Attraction of kerosene towards wick
15.For tap water and clean glass, the angle of contact is
a)0 degree
b)90 degree
c)140 degree
d)8 degree
Solutions:
- a)surface tension
- a) Reduction in the interfacial tension between the phases
- b)90 degree
- a)because the adhesion force between the water molecules and capillary wall is greater than the cohesion force between the water molecules
- a)surface tension is just balanced by the downward force of gravity due to the weight of the liquid
- b)hemispherical
- a)contact angle between the surface of the liquid and the capillary wall
- b)Stalagmometer
- c)W = mg = VǷg = πr2hǷg
- b) Immiscible liquids
- d)pycnometer
- d) All of these
- d) Atmospheric pressure
- a) Surface tension of oil
- d)8 degree
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 112-114.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 660-662.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test