METARAMINOL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
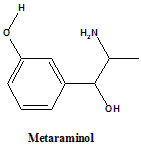
Metaraminol
IUPAC nomenclature
3-[(1R,2S)-2-amino-1-hydroxypropyl]phenol.
Classification
Metaraminol is a selective α1- adrenergic agonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 167.2 g/mol |
| 2 | Physical appearance | Present in solid form |
| 3 | Melting point | 107.5°C |
| 4 | Solubility | 1000g/L |
| 5 | Octanol/water partition coefficient | -0.27 |
| 6 | Presence of ring | Benzene ring |
| 7 | Number of chiral centers | 2 |
Mechanism of Action
i. Metaraminol acts as α1-drenergic agonist and produces peripheral vasoconstriction.
ii. It inhibits adenyl cyclase and thus, inhibition of the production of cAMP
iii. It also releases norepinephrine from its storage sites indirectly.
iv. All results in increase in systemic blood pressure. [1]
Structure Activity Relationship
The SAR of Adrenergic agonist can be discussed as follows:
- Primary or secondary aliphatic amine separated by two carbons from a substituted benzene ring is essential for the high agonist activity.
- The hydroxyl substituted carbon must be in the R configuration for the maximal direct activity.
R1 substitution:
- When R1 is increased in size, activity of alpha receptors decreases and activity of the beta receptors increases
- Activity of both alpha and beta receptors is maximum when R1 is methyl group.
- Alpha agonist activity decreases when R1 is larger than methyl, and went negligible when R1 is isopropyl.
- Large lipophillic groups can afford compounds with alpha blocking activity.
- N-substituent provides selectivity for different receptors.
- Arylalkyl group can provide beta selectivity, increased cell penetration and increased lipophillicity for the longer duration of action.
R2 substitution:
- Ethyl group can eliminate the alpha activity of the drug.
- Erythrostero isomers have maximal activity.
- The additional methyl group makes the drug more selective for the alpha2
R3 substitution on the aromatic ring:
- 3’,4’-dihydroxy substituted benzene ring has poor oral activity.
- 3’, 5’-dihydroxy compounds are orally active.
- At least one of the groups is required which can form hydrogen bonds. And if only one group is present then it is preferred at 4’ position to retain the beta2
If phenyl group has no phenolic substituent then it may act directly or indirectly. [2]
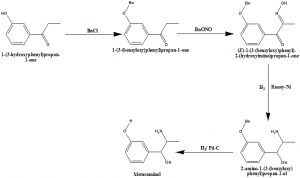
Method of synthesis
i. 1-(3-hydroxyphenyl)propan-1-one is reacted with chlorobenzene to give 1-(3-(benzyloxy)phenyl)propan-1-one.
ii. Reaction of the above compound with BnONO gives (E)-1-(3-(benzyloxy)phenyl)-2-(hydroxyimino)propan-1-one.
iii. When the latter compound undergoes reduction, it produces 2-amino-1-(3-(benzyloxy) phenyl)propan-1-ol.
iv. Latter compound further undergoes reduction to give Metaraminol.
Therapeutic Uses
Ephedrine is used:
- For prevention and treatment of acute hypotensive state occurring with spinal anesthesia.
- As an adjunctive treatment of hypotension due to hemorrhage
Side Effects
Side effects of metaraminol are:
- Sinus tachycardia
- Ventricular tachycardia
- Arrhythmias
- Tissue necrosis
- Nausea
- Vomiting
- Anxiety
- Dizziness
- Nervousness
MCQs
Q.1 Complete the given below sentence.
“Metaraminol acts on α-adrenergic receptors and leads to ……… of adenylcyclase, thus inhibition of production of ………. .
a) Stimulation, ATP
b) Inhibition, cAMP
c) Stimulation, cAMP
d) Inhibition, ATP
Q.2 Metaraminol is given to patient during?
a) Hypotension
b) Hypertension
c) Tachycardia
d) None of these
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug Metaraminol?
I. Primary or secondary aliphatic amine separated by two carbons from a substituted benzene ring is essential for the high agonist activity.
II. The hydroxyl substituted carbon must be in the R configuration for the maximal direct activity.
a) I
b) II
c) I, II
d) None
Q.4 The starting chemicals required for the synthesis of drug Metaraminol?
a) 1-(3-hydroxyphenyl)propan-1-one
b) 3-(4-nitrophenyl)butanone
c) Both a) and b) are required
d) None of these
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Metaraminol?
I. Molecular weight is 121.25 gm/mol
II. Its solubility is 1000 gm/L
III. Melts at room temperature.
IV. Cyclohexyl ring is present in the structure.
a) TFTF
b) TTFT
c) TTFF
d)FFTT
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Metaraminol: 3-[(1R,2S)-2-amino-1-hydroxypropyl]phenol
II. Pseudoephedrine: (S,S)-2-methylamino-1-phenylpropan-1-ol.
III. Hydroxyamphetamine: (RS)-[4-(1-Hydroxy-2-tert-butylamino-ethyl)-2-(4-methylbenzoyl)oxy-phenyl] 4-methylbenzoate
a) I, III
b) I, II
c) I, II, III
d) II, III
Q.7 Match the following drugs with their correct classifications-
| i. Metaraminol | A. Mixed-acting sympathomimetic |
| ii. Amphetamine | B. ß2-arenergic agonist |
| iii. Silodosin | C. Selective α1-adenergic agonist |
| iv. Pirbuterol | D. Selective α2-adrenergic antagonist |
a) i-D, ii-A, iii-C, iv-B
b) i-C, ii-A, iii-B, iv-D
c) i-B, ii-D, iii-C, iv-A
d) i-C, ii-A, iii-D, iv-B
ANSWERS
1-b
2-a
3-c
4-a
5-c
6-b
7-d
REFERENCES
[1] HARRISON DC, CHIDSEY CA, BRAUNWALD E. Studies on the mechanism of action of metaraminol (Aramine). Annals of internal medicine. 1963 Sep 1;59(3):297-305. [2] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 348-352