METHOTREXATE SAR, Synthesis, Mechanism of action, Therapeutic actions, Side effects and MCQ
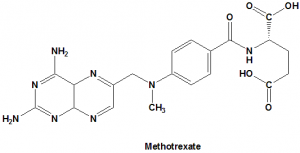
Methotrexate
IUPAC nomenclature
(2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid
Classification
Methotrexate falls under the category of Folate antagonist antimetabolite
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 454.4 g/mol |
| 2 | Appearance | Yellow to brown crystalline powder |
| 3 | Melting point | 195°C |
| 4 | Solubility | Freely soluble in chloroform, soluble in acetone |
| 5 | Octanol water partition coefficient | -1.85 |
| 6 | Presence of ring | Pteridine ring system, aromatic ring |
Mechanism of Action
i. Methotrexate competitively inhibits dihydrofolate reductase.
ii. The synthesis of tetrahydrofolate decreases.
iii. Due to this, the synthesis of nucleoside thymidine also decreases.
iv. This inhibits the synthesis of DNA.
v. Further, the purine synthesis is also inhibited by the methotrexate as the tetrahydrofolate is important for the synthesis of purine also. [1]
Structural Activity Relationship
- Replacement of glutamate tail with lipophillic agents can increase the transportation of the drug through folate carrier system.
- Thiourea entity can increase the activity of the drug
- Replacement of Thiazole with imidazol will increase the activity of the drug.
- Tetrahydroquinazolines derivatives will affect the ligand-enzyme interaction in a positive manner, where the dibenzodiazepine ring will show the pharmacophoric features which are essential for the activity of the drug.
- Substitution at 2nd, 3rd and 6th positions in the quinazolinone nucleus will inhibit the action of the drug.
- Substitution at ortho and para positions in phenyl ring will decrease the tendency of the drug to bind with DHFR, thus decreasing the activity of the drug.
- Combining the drug with copper metal can also increase the activity of the drug. [2]
Methods of Synthesis
i. Condensation of 2,3-dibromopropionaldehyde with 2,4,5,6-tetraaminopyrimidine to produce 6-bromomethyl-2,4-diaminopteridine.
ii. 6-bromomethyl-2,4-diaminopteridine will undergo further condensation with N-(para-(methylamino)benzoyl)glutamic acid. This will lead to the synthesis of methotrexate.

Therapeutic Uses
- Acute lymphoblastic leukemia
- Polyarticular juvenile idiopathic arthritis.
- Recalcitrant
- Disabling psoriasis
- Estational choriocarcinoma
- Chorioadenoma destruens
- Hydatiform mole
- Breast cancer
- Epidermoid cancer of the head and neck
- Advanced mycosis fungoides
- Lung cancer
- Advanced non-hodgkin’s lymphoma
- Acute lymphocytic leukemia.
Side Effects
- Weight loss, fevers and chills
- Vomiting and mouth sores
- Blood in the urine and with stools
- Shortness of breath
- Kidney problems like little or no urine formation
- Liver problems like jaundice
- Nerve problems like confusions, weakness, headache vision problems, etc.
- Signs of tumor cell breakdown
MCQs
Q.1 Which term is NOT associated with the drug methotrexate?
a) Neotrexate
b) (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid
c) Biotrexate
d) (2S)-2-[[4-[methyl-methylamino]benzoyl]amino]pentanedioic acid
Q.2 Match the following with respect to the physical forms of drugs
| i. Melaphalan | A. White to buff colored powder |
| ii. Methotrexate | B. Solid yellow powder |
| iii. Lomustine | C. White crystalline solid |
| iv. Busulfan | D. yellow to brown crystalline powder |
a) i-A, ii-D, iii-B, iv-C
b) i-B, ii-A, iii-C, iv-D
c) i-A, ii-C, iii-B, iv-D
d) i-D, ii-C, iii-A, iv-B
Q.3 The drug Methotrexate shows its action through
a) Alkylation of DNA
b) Inhibition of DNA synthesis
c) Aromatase inhibition
d) Antibiotic activity
Q.4 The correct order for the synthesis of the drug Methotrexate is?
I. Condensation of propionsulfonic acid with 2,4,5,6-tetraaminopyrimidine to produce 6-bromomethyl-2,4-diaminopteridine.
II. Condensation of 2,3-dibromopropionaldehyde with 2,4,5,6-tetraaminopyrimidine to produce 6-bromomethyl-2,4-diaminopteridine.
III. 6-bromomethyl-2,4-diaminopteridine will undergo further condensation with N-(para-(methylamino)benzoyl)glutamic acid. This will lead to the synthesis of methotrexate.
a) I-III
b) I-II
c) II-III
d) III-II
Q.5 Predict the incorrect statements from the following with respect to the classification of the drug.
I. Methotrexate belongs to the class of alkylating agents
II. Flutamide belongs to class antiandrogens
III. Dacarbazine is a Nitrosoureas alkylating agent
IV. Letrozole belongs to the class Aromatase inhibitors
a) I & III
b) I, II & IV
c) II & IV
d) I, II, III & IV
Q.6 The correct sequence of True and False for the given statements with respect to the drug Methotrexate is
I. It is used for the treatment of Cancers
II. It is used for the treatment of rheumatoid arthritis
III. It produces side effect like mouth sores and shortness of breath
IV. It is a purine antagonist
a) TTFF
b) TTTF
c) TFTF
d) FTFT
Q.7 Which amongst the following drugs is having highest number of ring system in its structure-
a) Lomustine
b) Melphalan
c) Busulfan
d) Methotrexate
ANSWERS
1-d
2-a
3-b
4-c
5- a
6- b
7- d
REFERENCES
[1] Cronstein BN. The mechanism of action of methotrexate. Rheumatic disease clinics of North America. 1997 Nov 1;23(4):739-55. [2] Raimondi MV, Randazzo O, La Franca M, Barone G, Vignoni E, Rossi D, Collina S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules. 2019 Jan;24(6):1140.