METHYSERGIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
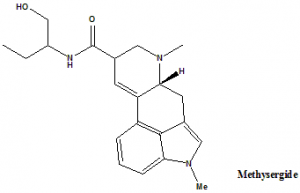
Methysergide
IUPAC nomenclature
(6aR,9R)-N-[(2S)-1-Hydroxybutan-2-yl]-4,7-dimethyl-6,6a,8,9-tetrahydroindolo[4,3-fg]quinoline-9-carboxamide.
Classification
Methysergide is an ergot alkaloid. It is a serotonin antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 353.5 g/mol |
| 2 | Physical appearance | Present in crystal solid form. |
| 3 | Melting point | 195°C |
| 4 | Solubility | Water solubility is 129 mg/L |
| 5 | Octanol/water partition coefficient | 1.55 |
| 6 | Presence of ring | Ergoline ring |
| 7 | Number of chiral centers | 2 |
Mechanism of Action
- It directly stimulates the CNS which stimulates the smooth muscles leads to vasoconstriction
- It also produces some α-adrenergic blockings. [1]
Structure Activity Relationship
- d-isomers of lysergic acid is inactive. It must be in the l-form.
- Antimigraine effect of the drug is due to the 9-10 double bond.
Method of synthesis
Methyl group and butanolamide group is added to the lysergic acid to give methysergide. [2]
Therapeutic Uses
Methysergide is used for:
- Migraine headaches
- Cluster headaches
- Prevention of migrains
Side Effects
Side effects of methysergide are:
- Retroperitoneal fibrosis
- Left-side cardiac valve dysfunction
MCQs
Q.1 “(6aR,9R)-N-[(2S)-1-Hydroxybutan-2-yl]-4,7-dimethyl-6,6a,8,9-tetrahydroindolo[4,3-fg]quinoline-9-carboxamide” is the IUPAC nomenclature of which drug?
a) Methysergide
b) Propanolol
c) Phenoxybenzamine
d) Tamsulosin
Q.2 Melting point of Methysergide is?
a) 124°C
b) 195°C
c) 221°C
d) 143°C
Q.3 Match the following with correct classifications of the drugs.
| i. Bisoprolol | A. Ergot alkaloid |
| ii. Methysergide | B. ß2-adrenergic agonist |
| iii. Isoproterenol | C. ß1-adrenergic antagonist |
| iv. Dobutamine | D. ß1-adrenergic agonist |
a) i-B, ii-C, iii-A, iv-D
b) i-D, ii-A, iii-C, iv-B
c) i-C, ii-A, iii-D, iv-B
d) i-C, ii-A, iii-B, iv-D
Q.4 Correct steps for the mechanism of action of the drug methysergide?
I. Vasoconstriction
II. Stimulation of smooth muscles
III. Stimulation of CNS
a) III – II – I
b) I – III – II
c) II – I – III
d) II – III – I
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug methysergide?
- d-isomers of lysergic acid is inactive. It must be in the l-form.
- Antimigraine effect of the drug is due to the 9-10 double bond.
a) TT
b) FT
c)TF
d)FF
Q.6 When methyl group and butanolamide groups are added to the lysergic acid, it produces?
a) Methamphetamine
b) Alfuzosin
c) Tetrahydrozoline
d) Oxymetazoline
Q.7 The drug Methysergide is mainly used for?
a) Hypotension
b) Hypertension
c) Migraine headache
d) None of these
ANSWERS
1-a
2-b
3-d
4-a
5-a
6-c
7-c