Micromeretics and powder rheology: Determining surface areas – Permeability method and Adsorption method and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
Specific surface: The specific surface of a powder is defined as the surface area per unit volume (Sv) or per unit weight (Sw).
SURFACE AREA DETERMINATION METHODS:
The surface area can be determined directly by one of the following two methods:
- Adsorption method
- Air permeability method
Adsorption Method – Particles with a small particle size (large specific surface) are good adsorbents for the adsorption of gases and of solutes from the solution. The amount of gas or solute adsorbed on the sample of powder to form a monolayer calculated, and from this data, the surface area of the powder is determined.
Solute adsorption method
Principle: Adsorption of a solute from its solution onto the surface of the adsorbent powder whose area is to be determined Here, solute refers to substance of known surface area, which gets adsorbed and forms a monolayer such as stearic acid and adsorbent powder refers to powder sample whose surface area is to be determined. It adsorbs the solute molecules.
- A solution of a known amount of solute (5.0 g) is first prepared in a medium in which the adsorbent powder is insoluble.
- A known amount of the adsorbent powder (0.5 g) is then added to the solution and the contents are stirred till equilibrium has been attained.
- The powder is then filtered and the amount of solute remaining in the solution (4.0 g) is determined by a suitable method.
- The difference between the quantity of solute added and that remaining in the solution gives the quantity of solute that has been adsorbed (1.0 g).
- From this value, the amount of solute adsorbed per gram of the powder is calculated.
Since the surface area of one molecule of solute is known, the specific surface area of the powder is calculated by the following equation:
Specific surface area of the power = number of molecules adsorbed × surface area of one molecule of solute
Gas adsorption method
Surface area determination by gas adsorption method is carried out using an instrument called quantasorb.
Method: The powders whose surface area is to be determined are introduced into a cell in the instrument and nitrogen (adsorbate gas) and helium (inert, nonadsorbing gas) are passed through the powder in the cell. A thermal conductivity detector measures the amount of nitrogen adsorbed at every equilibrium pressure and a bell-shaped curve is obtained on a strip-chart recorder. The signal height gives the rate of adsorption of nitrogen gas, and the area under the curve provides the amount of gas adsorbed on the powder sample. The volume of nitrogen gas Vm in cubic centimetre adsorbed by 1 g of the powder when the monolayer is given by the BET equation:
P / V(Ƿ0-Ƿ) = 1 / Vmb + (b-1)Ƿ / Vmb Ƿ0
where V is the volume of gas in cm3 adsorbed per gram of powder at pressure Ƿ.
Ƿ0 is the saturated vapour pressure of liquefied nitrogen at the temperature of the experiment.
b is a constant and it gives the difference between the heat of adsorption and the heat of liquefaction of the nitrogen gas.
A plot of Ƿ /V(Ƿ0– Ƿ) versus p/po generally gives a straight line. The slope and intercept yield the values b and Vm, respectively.
The specific surface of the powder is obtained by applying the following equation:
Sw = AmN X Vm / (m/p)
where m/p is the molar volume of the gas, which is equal to 22,414 cm3/mol at NTP. N is Avogadro’s number 6.02 × 1023. Am is the area of a single close packed gas molecule absorbed as a monolayer on the surface of the powder particles. For nitrogen, the value is 16.2 × 10 -16 cm2.
Air Permeability Method:
Principle: This method is based on the principle that the resistance offered to the flow of a fluid, such as air, through a plug of compacted powder is proportional to the surface area of the powder. The greater the surface area per gram of the powder, the greater is the resistance to flow. Surface area determination by the air permeability method is generally carried out with an instrument called the Fisher subsieve sizer.
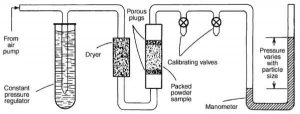
Fig 1 – Representation of Fisher subsieve sizer apparatus (taken from Fisher subsieve sizer powder metallurgy)
Method:
- Powder is packed in sample holder.
- Packing appears as series of capillaries.
- Air is allowed to pass through the capillaries at constant pressure.
- Resistance is created as air passes through capillaries thus causing pressure drop.
- Greater the surface area greater the resistance.
- Air permeability is inversely proportional to the surface area.
According to Poiseuille’s equation:
V = πd4∆pt / 128lƞ
where V is the volume of air flowing through a capillary of internal diameter d and length l in t seconds under a pressure difference of ‘P. The viscosity of the fluid (air) is K poise.
When the air is allowed to pass through the plug of a compacted powder, resistance to the flow of air occurs. This resistance is related to the surface area of the powder. According to the Kozeny–Carman equation derived from Poiseuille’s equation:
V = A/ƞSw2 X ∆p/kl X E3/ (1-E)2
where A is the cross-sectional area of the plug, K is a constant (usually 5.0 ± 0.5) and E is the porosity.
Multiple choice questions (MCQs)
1.___ is not the fundamental property of powders
a)Particle shape
b)Particle volume
c)Particle number
d)Bulk density
2.____ is not the derived property of powders
a)Particle shape
b)Tapped density
c)Bulk density
d)Carr’s index
3.Equivalent spherical diameter of asymmetric particle can be calculated using
a)Surface area
b)Volume
c)Diameter
d)All of the above
4.Surface area of spherical particle is given by
a)Πd
b)Πd2
c)Πd3
d)Πd4
___ diameter is the diameter of sphere having the same surface area as that of the asymmetric particle in question
a)Surface
b)Projected
c)Volume
d)None of these
6.___ diameter is the diameter of sphere having the same surface area as that of the asymmetric particle in question when viewed in its most stable plane
a)Surface
b)Projected
c)Volume
d)None of these
7.___ diameter is the diameter of sphere having the same density as that of the asymmetric particle in question and which undergoes sedimentation at the same rate as the asymmetric particle
a)Surface
b)Projected
c)Volume
d)Stokes
8.When the ____ of particle is plotted against the mean particle size, the curve obtained is called as number frequency distribution curves
a)Number
b)Volume
c)Area
d)Thickness
9.When the ____ of particle is plotted against the mean particle size, the curve obtained is called as weight distribution frequency curve
a)Number
b)Weight
c)Area
d)Thickness
10.Bell shape curve obtained by plotting frequency verses logarithm of particle diameter is referred as log normal distribution curve
a)Number distribution frequency curve
b)Weight distribution frequency curve
c)Log normal distribution curve
d)None of these
11.When the log particle size is plotted against the cumulative percent frequency on a probability scale showing linear relationship is called as
a)Log probability plot
b)Weight distribution frequency
c)Log normal distribution curve
d)None of the above
12.___ can be characterized by two parameters that is the slope of the line and a reference point
a)Log probability plot
b)Weight distribution frequency
c)Log normal distribution curve
d)None of the above
13.____ is the logarithm of particle size equivalent to 50% on the probability scale (that is the 50% size)
a)Reference point
b)Standard point
c)Freezing point
d)Boiling point
14.___ is defined as the number of particles per unit weight of a powder
a)Particle weight
b)Particle frequency
c)Particle number
d)All of the above
15.____ is not the method of determination of particle size
a)Microscopic method
b)Sieving
c)Sedimentation
d)Melting
Solutions:
- d) Bulk density
- a)Particle shape
- d)All of the above
- b)Πd2
- a)Surface
- c)Volume
- d)Stokes
- a)Number
- b)Weight
- c)Log normal distribution curve
- a)Log probability plot
- a)Log probability plot
- a)Reference point
- c)Particle number
- d)Melting
References:
1. GAURAV KUMAR JAIN – THEORY & PRACTICE OF PHYSICAL PHARMACY, 1st edition 2012 Elsevier, page no. 44-47.
2. Martins Physical Pharmacy, 6th edition 2011, page no. 825-828.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE