MORPHINE Synthesis, SAR, MCQ and Chemical Structure
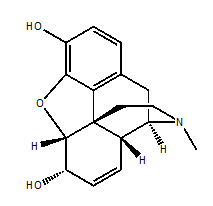
Morphine
IUPAC NOMENCLATURE: (4R,4aR,7S,7aR,12bS)-3-Methyl-2,3,4,4a,7,7a-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diol. [1]
CLASSIFICATION: Morphine is an opiate analgesic and narcotic drug.
PHYSIOCHEMICAL PROPERTIES:
1. Molecular weight : 285.34 g/mol
2. Appearance : White crystalline solid
3. Melting point : 255°C
4. Solubility : 149 mg/L in water at 20 °C
5. Dissociation constant (pKa) : 8.21 at 25°C
6. Presence of ring : Phenolic ring, cyclohexane ring, N-methyl-piperidine ring and partially saturated furane ring.
MECHANISM OF ACTION:
- Morphine acts as opioid receptor agonist in the brain.
- Thereby, it shows action by coupling with calcium channels with the help of G proteins.
- This results in producing the calcium channel antagonist properties of morphine.
- Other than this, Morphine also binds to postsynaptic receptors and hyperpolarizes postsynaptic neurons.
- All these actions together helps in reducing the pain signals to the CNS. [2]
STRUCTURAL ACTIVITY RELATIONSHIP
- Replacement of phenolic hydroxyl into –OCH3/-OC2H5 will make the morphine less analgesic and cough suppression will also takes place.
- Replacement of alcoholic hydroxyl with –OCH3 makes the compound 5 times more active.
- Replacement of alcoholic hydroxyl with -OC2H5 makes the compound 2.4 times more active than morphine.
- Replacement of alcoholic hydroxyl with –OCOCH3 will also activates the compound by 4.2 times.
- Replacement of alcoholic hydroxyl with ketone group inactivates the compound and makes it lesser active.
- By hydrogenation of alicyclic unsaturated linkage, activity increases by 1.2 times.
- On replacement of the methyl group from tertiary nitrogen by hydrogen atom, activity decreases.
- On replacement of N-CH3 by NCH2CH2Ph, activity increases by 14 times.
- When the methyl group of tertiary nitrogen replaced by N-allyl/methallyl/propyl, the compound so formed acts like the Morphine antagonist.
- When the methyl group of tertiary nitrogen replaced by N(CH3)2 Cl– , compound have curare action and it do not possesses any analgesic activity. [3]
METHOD OF PRODUCTION
Opium plants are crushed and diluted in the sulfuric acids. This helps in the extraction of opioids from poppy plants.
Extract is collected in the form of precipitate by reacting it with ammonium hydroxide solution.
Purification of product is done to separate the morphine from other opioids products.
THERAPEUTIC USES:
- For the reduction of both, acute and chronic pain
- In reducing the symptoms of shortness of breath.
- Also used as an opiate substitution therapy.
SIDE EFFECTS
- Constipation
- Hormone imbalance
- Impairment of human performance
- Addiction
- Development of tolerance of the opioids in the brain cells
- Person become dependent on the Morphine
MULTIPLE CHOICE QUESTIONS
Q(A) (4R,4aR,7S,7aR,12bS)-3-Methyl-2,3,4,4a,7,7a-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diol is the IUPAC name of?
1. Tinidazole
2. Morphine
3.Penicillin
4. Promidazole
(B)Which type of ring is NOT present in morphine structure?
1. Phenolic
2. Cyclohexane
3. Furane
4. Imidazole
(C)Morphine is obtained from
1.Poppy plant
2.Penicillium
3Rauwolfia
4Both 1 and 3
(D)Morphine is used for-
1.Reducing the acute pain
2.Reducing the chronic pain
3.Treatment of hotness of breath
4.All of the above
(E) Which statement/s is/are correct?
I. Morphine intake can produce addiction
II. Morphine is an opioid drug
III. Morphine can help in reducing the chronic pain
1.I,II and III are correct
2.II and III are correct
3.II is incorrect
4.Only I is correct
(F) On replacement of N-CH3 by NCH2CH2Ph, activity changes by?
1.Activity remains constant
2.Activity increases 2 times
3.Activity decreases by 2 times
4.Activity increases by 14 times
(G) Which statement is correct?
1.Replacement of alcoholic hydroxyl with –OCH3 makes the compound more active than morphine
2.Replacement of alcoholic hydroxyl with –OCH3 makes the compound less active than morphine
3.Replacement of alcoholic hydroxyl with –OCH3 makes the compound will have no effect on its activity on morphine
4.Replacement of phenolic hydroxyl with –OCH3 makes the compound more active than morphine
ANSWERS
(A). (1) (4R,4aR,7S,7aR,12bS)-3-Methyl-2,3,4,4a,7,7a-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7,9-diol is the IUPAC name for morphine.
(B)(4) Phenolic ring, cyclohexane ring, N-methyl-piperidine ring and partially saturated furane rings are present in morphine
(C)(2) Morphine is obtained by fungi poppy plant.
(D). (4) morphine can be used for reducing chronic and acute pains, and also is helpful in treatment of symptoms like shortness of breath.
(E). (1) all the options are correct for morphine.
(F). (4) activity will increase by 14 times
(G). (1) Replacement of alcoholic hydroxyl with –OCH3 makes the compound 5 times more active.
REFERENCES
[1] Small L, Rapoport H. Nuclear substituted morphine derivatives. The Journal of organic chemistry. 1947 Mar;12(2): pp.284-92.
[2] Tripathi KD. Essentials of medical pharmacology. JP Medical Ltd; 2013 Sep 30.: pp. 378-390.
[3] Wilson CO, Beale JM, Block JH. Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. Baltimore, MD: Lippincott Williams & Wilkins,; 2011: pp.783-784.