PHENTOLAMINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Phentolamine
IUPAC nomenclature
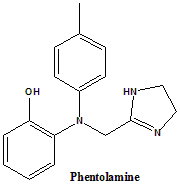
3-[(4,5-Dihydro-1H-imidazol-2-ylmethyl)(4-methylphenyl)amino]phenol.
Classification
Phentolamine is nonselective α-adrenergic antagonist. It can also be categorize as an antidote.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 281.35 g/mol |
| 2 | Physical appearance | Present in crystalline solid form |
| 3 | Melting point | 174.5°C |
| 4 | Solubility | 4.59 mg/L in water |
| 5 | Octanol/water partition coefficient | 3.3 |
| 6 | Presence of ring | Benzene and imidazoline rings are present |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
i. Phentolamine competitively blocks α-adrenergic receptors.
ii. It leads to relaxation of muscles and widening of blood vessels.
iii. Widening of blood vessels results in decrease in blood pressure.
Phentolamine also stimulates ß-adrenergic receptors and produces a positive inotropic and chronotropic effect on the heart which results in increase in the cardiac output. [1]
Structure Activity Relationship
- Molecules with 2,6-disubstitutions, which assume an orientation where the phenyl and imidazoline rings are in different plans are having the a highest activity.
- Electronic effects have only influence on the actions at H2; action on α-receptors are not influenced.
- Substitutions at 3, 4 or 5 positions of the phenyl ring preclude potent activity at H2-receptor sites while the α-receptor activity is maintained. [2]
Method of synthesis
m-(p-toluidino)phenol is refluxed with 2-chloromethylimidazoline to give phentolamine.

Therapeutic Uses
Phentolamine is used for:
- Reversing numbness after dental procedures
- For the prevention of the events of high blood pressure
- Treatment of accidental injection of certain drugs under the skin
- Diagnosis of pheochromocytoma
Side Effects
Side effects of Phentolamine are:
- Pain at injection site
- Change in heart rate
- Headache
- Weakness
- Diarrhea
- Redness and warmth in skin
- Vomiting
- Nausea
- Dizziness
MCQs
Q.1 What can be the correct IUPAC nomenclature of Phentolamine
a) 3-[(4,5-Dihydro-1H-imidazol-2-ylmethyl)(4-methylphenyl)amino]phenol
b) 3-[(1R,2S)-2-amino-1-hydroxypropyl]phenol
c) 2-(naphthalen-1-ylmethyl)-4,5-dihydro-1H-imidazole
d) (RS)-4-(2-{[4-(4-hydroxyphenyl)butan-2-yl]amino}ethyl)benzene-1,2-diol
Q.2 Which amongst the following statements is/are correct related to the SAR of phentolamine?
I. Molecules with 2,6-disubstitutions, which assume an orientation where the phenyl and imidazoline rings are in different plans are having the a highest activity.
II. Electronic effects have only influence on the actions at H2; action on α-receptors are not influenced.
III. Substitutions at 3, 4 or 5 positions of the phenyl ring preclude potent activity at H2-receptor sites while the ↑-receptor activity is maintained.
a) I, II
b) I, II, III
c) II, III
d) I
Q.3 m-(p-toluidino)phenol is refluxed with 2-chloromethylimidazoline to give?
a) Dobutamine
b) Sildosin
c) Phentolamine
d) Mthacholine
Q.4 Side effects of drug phentolamine is/are?
a) change in heart rate
b) Headache
c) Dizziness
d) All of the above
Q.5 Match the following drugs with their correct melting points-
| i. Phentolamine | A. 184-186°C |
| ii. Dobutamine | B. 174.5°C |
| iii. Metaraminol | C. 257°C |
| iv. Naphazoline | D. 107.5°C |
a) i-D, ii-B, iii-A, iv-C
b) i-A, ii-D, iii-C, iv-B
c) i-C, ii-B, iii-D, iv-A
d) i-B, ii-A, iii-D, iv-C
Q.6 An example of drug from class nonselective α-adrenergic antagonist?
a) Metaraminol
b) Dopamine
c) Albuterol
d) Phentolamine
Q.7 The type of ring system found in Phentolamine?
a) Imidazoline
b) Ergorine
c) Cyclopropane
d) None of the above
ANSWERS
1-a
2-b
3-c
4-d
5-d
6-d
7-a
REFERENCES
[1] Gould LA, Zahir MO, Ettinger ST. Phentolamine and cardiovascular performance. British heart journal. 1969 Mar;31(2):154. [2] Malta E, Ong JS, Raper C, Tawa PE, Vaughan GN. Structure-activity relationships of clonidine-and tolazoline-like compounds at histamine and alpha-adrenoceptor sites. British journal of pharmacology. 1980 Aug;69(4):679.