POTENTIOMETRY PART -2- GPAT, GATE, UGC NET JRF Notes
REFERENCE ELECTRODES :-
2. SATURATED CALOMEL ELECTRODES:-
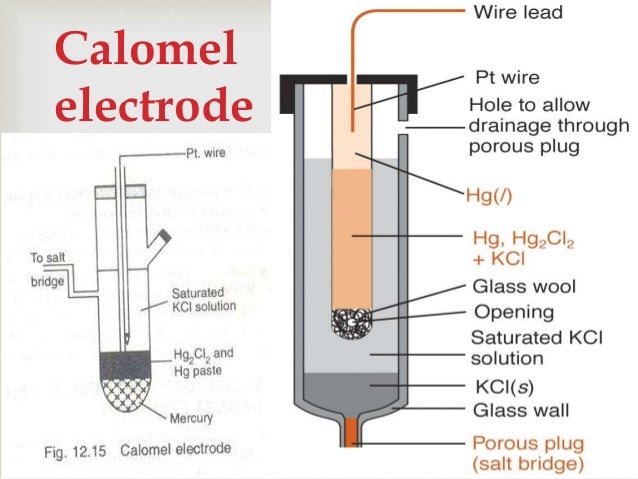
It is consists of an inner jacket and outer sleeve. Inner tube has wire contact with mercury and plugged with a mixture of calomel and KCL.
Outer sleeve is filled with saturated KCL of 1N or 0.1N KCL.
POTENTIAL OF CELL DEPENDS UPON
- Concentration of KCL
- Temperature
Demerits:-
- Unstable at high temperature
- Unstable where chloride ions show incompatibility
REACTION:-
- IF REDUCTION TAKE PLACE:-
Hg2Cl2→ Hg2+2 +2cl–
2. IF OXIDIATION TAKE PLACE:-
2Hg +2e– → Hg2+2
3. silver-silver chloride electrode Ag-Agcl :-
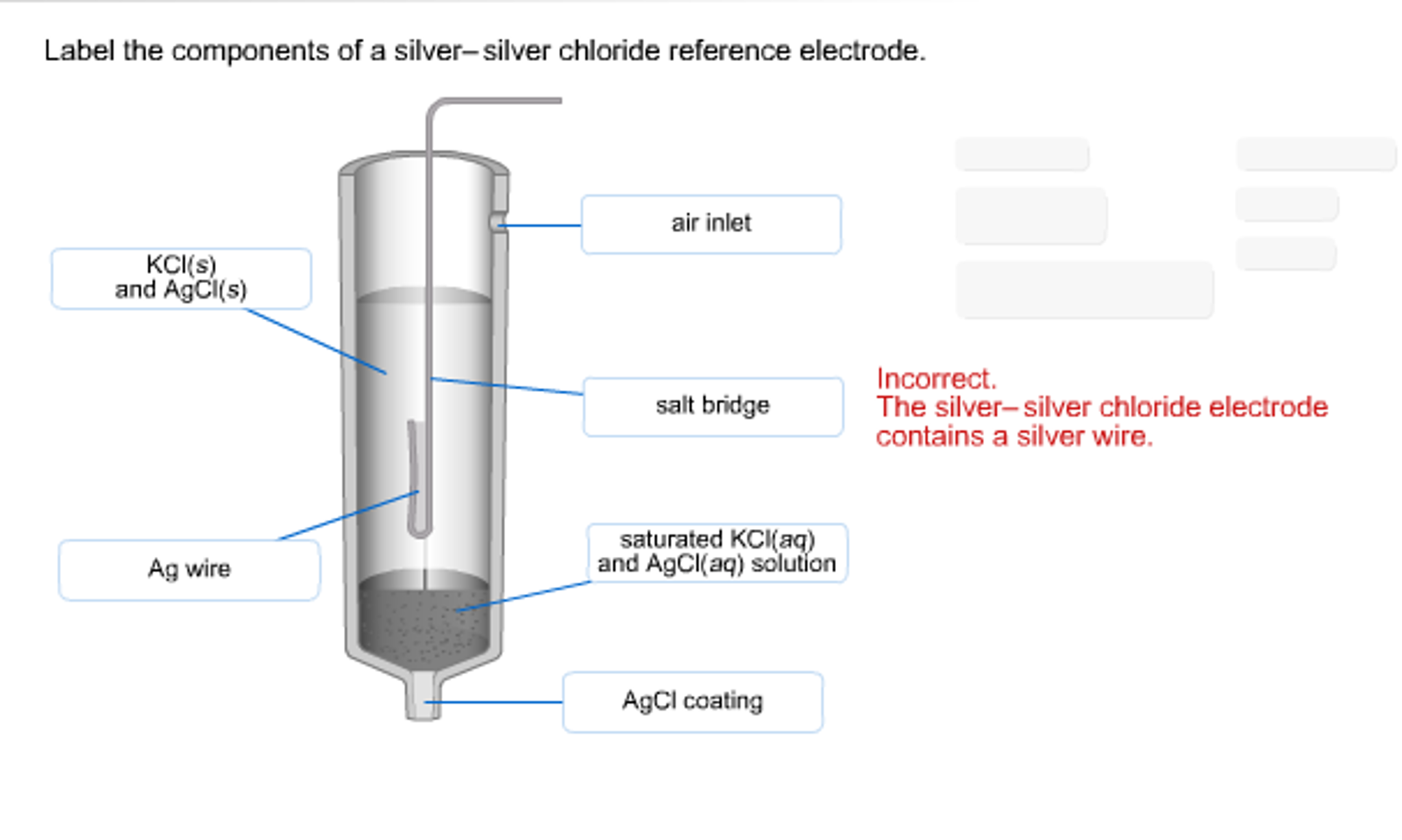
It is simply a silver wire coated electrolytically with silver chloride and dipped into potassium chloride. The potential of this half cell also depends on temp. as well as concentration of potassium chloride used.
| silver-silver chloride electrode used with | potential in at 25 c |
| saturated KCL | 200 |
| 1N KCL | 235.5 |
| 0.1N KCL | 288 |
REACTION:-
If reduction take place
Ag+ + e– ⇒ Ag
If oxidation take place
Ag⇒ Ag+ +e–
merits:-
it is easy to use.
demerits:-
it is difficult to prepare.
INDICATOR ELECTRODES :-
Indicator electrode is used in conjugation with reference electrode.It is used to determine the concentration of analyte in the sample solution.
type of indicator electrodes :-
- Noble metal electrode e.g :- Pt or AU
- First kind metal indicator Eg :- cu for cuso4 , zn for znso4
- Second kind metal indicator eg:- Ag will be coaked with AgCl.
- Oxide Electrode eg :- Sb sb2o3 ,
GLASS ELECTRODE :-
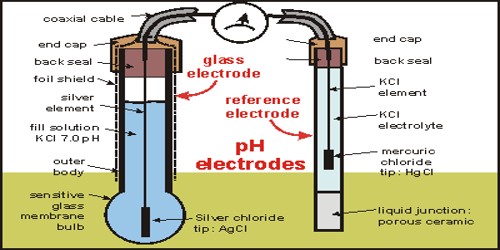
Glass electrode is the most widely used indicator electrode. It is selective and responsive to change in concentration of hydrogen ions. The glass electrode consists of a glass tube with a thin pH sensitive glass bulb at its tip. It has a silver-silver chloride wire at the centre of the tube and the lower tip of the wire at the centre of the tube and the lower tip of the wire immerses into the 0.1 N HCl filled in the glass tube. This assembly acts as an indicator electrode and is dipped into a solution whose pH or potential is to be known. it is
COMPOSITION :-
22% Na2O, 6%Cao, 72%Sio2
MERITS:-
- Gives a rapid response.
- Chemically resistant to oxidizing, reducing agents, dissolved gas,colloids & salts.
- When lithia-silica glasses used it can be used over entire pH range.
DEMERITS :-
- Not used with simple potentiometer because of high internal resistance.
- > 9 pH other ion may transfer.
CATION SELECTIVITY : H> K> Na >NH4
2. HYDROGEN ELECTRODES :- Hydrogen electrodes has been describe in details under reference electrode. It is used as reference electrodes when dipped into a standard acid solution. Hydrogen electrode is used as indicator electodes when dipped into a unknown solutions whose potential or pH has to be determined.
3.Sb-Sb2O3 electrode :(also used in pH measurement)
It consists of a Sb rod coated with Sb2O3.
merits:-
- can be used with viscous and turbid solution.
- not easily poisoned or damaged.
- can be used from pH 3 to 8.
Demerits:-
- Not in presence of dissolved O2, oxidizing & complexing agents highly acidic or alkaline condition solution containing Ag,Au,Cu,noble metal.
- suffer from salt of error.
4. MEMBRANE / ION SELECTIVE ELECTRODES :-
The glass electrode can be modified suitably to a selective ion/membrane electrode. the composition of the membrane is changed by adding specific ions or synthetic substance or polymer thereby the electrode can be used for measuring cation or anions. for example,
- cation selective glass electrode is used in the determination of H+, Li+,Na+,Ag+or K+
- Divalent cation activity electrode can be used to measure total hardness of water and for complexometric titration using EDTA.
- Heterogenous membrane or soild state electrode can be used measure concentration Cl–,Br–,I–,CN–,S-2 etc.
POTENTIOMETRIC TITRATIONS :-
PRINCIPLE:-
These are titration in which the end point of titration can be determined by measuring the potential of changes in the potential of a solution caused by the addition of titrant. Alternatively the pH can also be monitored during the titration to detect end point. In a titration , the additing of titrant causes changes in the concentration or activity of ions in solutions.
INSTRUMENTATION:-

various part of potentiometer :-
1.electrodes :-
- REFERENCE ELECTRODE :-
- Standard hydrogen electrode
- saturated calomal electrode
- Ag-Agcl electrode
- INDICATOR ELECTRODE :-
- Glass membrane electrode
- metal electrode
2.TITRATION VESSELS :-
- contains sample analyte.
3.Titration Burette :-
- standard solution will be present.
4.magnetic stirrer.
MCQ.
1.Which is not a reference electrodes?
a.hydrogen electrodes
b.saturated calomel electrodes
c.Ag-Agcl electrodes
d.Glass electrodes
2.which is right composition of glass electrode?
a.22% Na2O, 6%Cao, 70%Sio2
b.22% Na2O, 8%Cao, 72%Sio2
c.22% Na2O, 6%Cao, 72%Sio2
d.21% Na2O, 6%Cao, 72%Sio2
3.Which factor is affecting SATURATED CALOMEL ELECTRODES ?
a.Concentration of KCL
b.Temperature
c.a and b
d.none of the above
4.which is example of first kind metal indicator ?
a.Pt electrode
b.AU electrode
c. glass electrode
d.zn or znso4 electrode
5. which is dis advantage of glass electrode ?
a.Gives a rapid response.
b.Chemically resistant to oxidizing, reducing agents, dissolved gas,colloids & salts.
c.When lithia-silica glasses used it can be used over entire pH range.
b.Not used with simple potentiometer because of high internal resistance
ANSWER KEY :-
1.d
2.c
3.c
4.d
5.d
REFERENCE :-
Text book of pharmaceutical analysis third edition of dr.s.ravi sankar (9.3-9.17)