PREDNISOLONE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Prednisolone
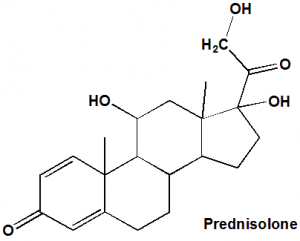
IUPAC nomenclature
(11β)-11,17,21-Trihydroxypregna-1,4-diene-3,20-dione.
Classification
Prednisolone falls under the category of glucocorticoids. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 360.4 g/mol |
| 2 | Appearance | White crystalline powder. Prednislolone have one sesquihydrate and two polymorphic forms (I & II). Form I is present in the monoclinic space while the other two forms exist in orthorhombic form. |
| 3 | Melting point | 235°C |
| 4 | Solubility | 223 mg per liter in water |
| 5 | Octanol/water partition coefficient | 1.62 |
| 6 | Presence of ring | Phenanthrene and cyclopentane |
| 7 | Number of chiral centers | 7 |
Mechanism of Action
- Prednisolone decreases vasodilation and permeability of capillaries. It also decreases the migration of the leukocytes to the site of inflammation. Prednisolone binds with glucocorticoid receptor which results in the change in the gene expression which leads to the multiple downstream effects.
- Prednisolone also inhibits demargination and neutrophil apoptosis. The drug decreases the formation of arachidonic acid derivatives by inhibition of phospholipase A2.
- It inhibits NF-Kappa B and some other inflammatory transcription factors and also promotes anti-inflammatory genes like interleukin-10.
- Lower doses of Prednisolone acts as anti-inflammatory drug, while higher doses acts as immunosuppressive drug. if higher doses of this drug taken for longer period of time, then it may results in the raise in the sodium levels and decrease in the potassium level.
Structural Activity Relationship
- Oxygen at C11 is important for the activity of drug.
- Alcoholic oxygen at C11 is superior than the ketonic one.
- Introduction of 17-alpha-hydroxy group increases the activity.
- Introduction of 6-alpha-F or 9-alpha-F group can also increase the activity of drug.
- Double bond at C1 position increases anti-inflammatory activity of the drug. [1]
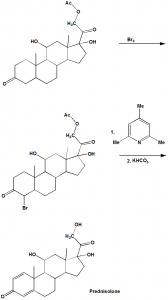
Method of synthesis
i. 21-acetoxy-11β,17α-dihydroxy-5α-pregnan-3,20-dione undergoes dibromination at positions C-2 and C-4.
ii. The formed dibromide is dehydrobrominated by heating it in collidine to obtain prednisolone as an acetate at position C-21.
iii. Hydrolyzing the latter product gives Prednisolone.
Therapeutic Uses
Prednisolone is given for the treatment of
- Inflammations in various parts of body
- Allergic reactions
- Autoimmune diseases
- Skin diseases
- Asthma
- Leukemia
- Lymphoma
- Multiple myeloma
- Nausea
- Vomiting
Drug is also used for the stimulation of the appetite in cancer patients.
Side Effects
- Common side effects of Prednisolone include increases appetite, rise in blood sugar level, impaired wound healing, heartburn, swelling in ankles, insomnia and irritability.
- Less common side effects are headache, cataracts, bone thinning and dizziness.
MCQs
Q.1 ‘(11β)-11,17,21-Trihydroxypregna-1,4-diene-3,20-dione’ is the IUPAC nomenclature of which drug?
a) Imatinib
b) Carmustine
c) Thiotepa
d) Prednisolone
Q.2 Predict the incorrect statement related to the therapeutic uses of drug Prednisolone-
a) It is given to stimulate the appetite of the patient
b) It is given for the treatment of cancers
c) It is given to induce vomiting in patients
d) It is given for the treatment of autoimmune disorders
Q.3 Match the following with respect to the SAR of drug prednisolone-
| i. Introduction of 17-alpha-hydroxy group | A. Increases the activity of drug |
| ii. Double bond at C-1 position | B. Decreases the activity of drug |
| C. Increases the activity of drug | |
| D. Decreases the activity of drug |
a) i-A, ii-C
b) i-A, ii-D
c) i-B, ii-C
d) i-B, ii-D
Q.4 Which amongst the following drugs shows its effect through inhibition of phospholipase A2?
a) Prednisolone
b) Imatinib
c) Mechlorethamine
d) Flutamide
Q.5 Prednisolone drug belongs to which class?
a) Estrogen antineoplastic drug
b) Glucocorticoid antineoplastic drug
c) Progestin antineoplastic drug
d) Antiandrogen antineoplastic drug
Q.6 Which of the following is NOT a side effect of Prednisolone?
a) Loss of appetite
b) Muscle weakness
c) Insomia
d) Increases blood sugar level
Q.7 How many ring are present in the structure of Prednisolone?
a) 1
b) 2
c) 3
d) 4
ANSWERS
1-d
2-c
3-a
4-a
5-b
6-a
7-d
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Gutterer B, inventor; Takeda GmbH, assignee. Prednisolone derivativesUnited States patent US 5,733,901. 1998 Mar 31.