PROCARBAZINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Procarbazine
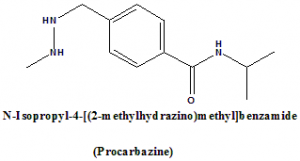
IUPAC nomenclature
N-Isopropyl-4-[(2-methylhydrazino)methyl]benzamide
Classification
Procarbazine falls under the category of Antineoplastic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 221.3 g/mol |
| 2 | Appearance | Present in solid form |
| 3 | Melting point | 223°C |
| 4 | Solubility | 1400 mg in 1 liter water |
| 5 | Presence of ring | Phenyl ring present |
Mechanism of Action
i. Transmethylation of methyl group of methionine into t-RNA is inhibited by the drug procarbazine.
ii. This causes the cessation of protein synthesis, which further leads to the cessation of DNA and RNA synthesis addition.
iii. Procarbazine can also directly attack the DNA by attacking on the protein sulfahydral groups contained in residual protein which is tightly bound to DNA. [2]
Structural Activity Relationship
- Binding with the amino group will increase the oral route availability of the drug
- The introduction of the substituted phenyl group will also increases the oral route availability of the drug.
- Aromatic ring introduction will increase the stability of the drug.
- Aromatic ring will further increase the distribution of the drug throughout the body.
- Benzimidazole ring can provide the local and faster action of the drug.
- Benzimidazol will further decrease the half life of compound.
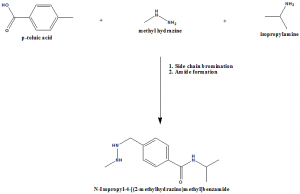
Methods of Synthesis
p-toluic acid, methyl hydrazine and isopropylamine are reacted together for the side chain bromination and amine/amide formation.
Therapeutic Uses
Procarbazine is used for the treatment of:
- Hodgkin’s disease
- Non-Hodgkin’s lymphoma
- Multiple myeloma
- Lung cancer
- Melanoma
- Brain tumors
Side Effects
- Common side effects include Low WBC count, low platelet count, nausea, vomiting and poor appetite.
- Some people may suffer from side effects like infertility, hypersensitivity reactions, central neurotoxicity, flu-like symptoms, constipation, diarrhea, mouth sores and hair loss.
MCQs
Q.1 What can be the correct IUPAC nomenclature of Procarbazine drug?
a) N,N-Dipropyl-4-[(2-methylhydrazino)methyl]benzamide
b) (SP-4-2)-diamminedichloroplatinum(II)
c) (SP-4-2)-diamminedichloroplatinum(I)
d) N-Isopropyl-4-[(2-methylhydrazino)methyl]benzamide
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Procarbazine?
I. Binding with the amino group will decrease the oral route availability of the drug
II. The introduction of the substituted phenyl group will also increases the oral route availability of the drug
III. Benzimidazole ring can provide the local and faster action of the drug.
a) I & II
b) I only
c) I, II, & III
d) All statement s are true
Q.3 The correct order for the mechanism of action of procarbazine can be?
I. Cessation of protein synthesis
II. Transmethylation of methyl group of methionine into t-RNA is inhibited
III. Cessation of DNA and RNA synthesis addition.
a) II – III – I
b) I – II – III
c) III – II – I
d) III – I – II
Q.4 The drug Procarbazine is mainly used for?
a) Hyperuricemia
b) Bone marrow depression
c) Treatment of Cancers
d) The immunosuppression
Q.5 Match the drugs with the correct classification.
| i. Procarbazine | A. Nitroimidazole |
| ii. Satranidazole | B. Alkaloids |
| iii. Emetine | C. Luminal amoebicides |
| iv. Tetracycline | D. Antineoplastic cytotoxic |
a) i-D, ii-A, iii-B, iv-C
b) i-C, ii-B, iii-A, iv-D
c) i-C, ii-B, iii-D, iv-A
d) i-C, ii-A, iii-B, iv-D
Q.6 How many statements below are true with respect to the side effects of the drug Procarbazine?
- Flu like symptoms
- Infertility
- Slight risk of developing blood cancer
- Hair loss
a) 1
b) 2
c) 3
d) 4
Q.7 The type of ring system found in Procarbazine is?
a) Imidazole ring
b) Phenyl ring
c) Pteridine ring
d) None of the above
ANSWERS
1-d
2-d
3-a
4-c
5-a
6-d
7-b
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Kreis W. Mechanism of action of procarbazine. InProceedings of the Chemotherapy Conference on Procarbazine (Matulane NSC-77213): Development and Application 1971 (pp. 35-44). US Government Printing Office.GPAT or Competitive Exam Aspirants can comment in comment box