PROCYCLIDINE HYDROCHLORIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
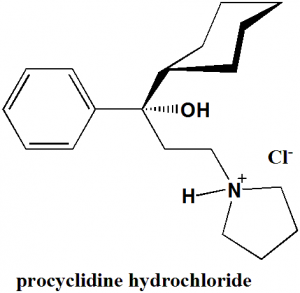
Procyclidine hydrochloride
IUPAC nomenclature
1-cyclohexyl-1-phenyl-3-pyrrolidin-1-ylpropan-1-ol;hydrochloride.
Classification
Procyclidine is an acetylcholine antagonist. It is a muscarinic antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 323.9 g/mol |
| 2 | Physical appearance | White crystalline substance |
| 3 | Melting point | 86°C. |
| 4 | Solubility | Soluble in water |
| 5 | Octanol/water partition coefficient | 4.2 |
| 6 | Presence of ring | Benzene, pyrrolidine, cyclohexane |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
- Procyclidine blocks the central cholinergic receptors and balances the cholinergic and dopaminergic activity in the basal ganglia.
- It is also having pharmacological similarities with atropine, which results in many of its effects.
- It also exerts an antispasmodic effect on smooth muscle, and results in the production of mydriasis and reduction in salivation. [1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potent derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
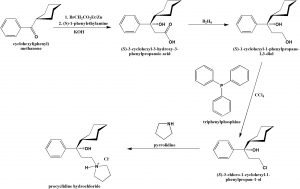
Method of synthesis
i. cyclohexyl(phenyl) methanone reacts with ethyl-2-bromoacetate and (S)-1-phenylethylamine to give (S)-3-cyclohexyl-3-hydroxy-3-phenylpropanoic acid.
ii. The latter compound is reacted with B2H6 to give (S)-1-cyclohexyl-1-phenylpropane-1,3-diol.
iii. On reaction with triphenylphosphine, (S)-3-chloro-1-cyclohexyl-1-phenylpropan-1-ol ca be obtained.
iv. Ontreating with pyrrolidine, procyclidine hydrochloride is produced. [1]
Therapeutic Uses
Procyclidine hydrochloride is used for:
- Treatment of Parkinson’s disease
- Treatment of side effects of antipsychotics
Side Effects
Side effects of procyclidine are:
- Dry mouth
- Dizziness
- Constipation
- Drowsiness
- Allergic reactions
- Blurred vision
MCQ
Q.1 Correct statements related with the Physicochemical properties of Procyclidine hydrochloride are?
I. Molecular weight = 496 gm/mol
II. Physical appearance: present in white crystalline substance form
III. Melting point = 199.5°C
IV. Solubility: soluble in water
a) I, III
b) I, II, IV
c) II, IV
d) I
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Methacholine | A. 2-(Acetyloxy)-N,N,N-trimethylpropan-1-aminium. |
| ii. Homatropin | B. (RS)-(8-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate |
| iii. Procyclidine | C. (N-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 2-hydroxy-2-phenylacetate |
| iv. Atropin | D. 1-cyclohexyl-1-phenyl-3-pyrrolidin-1-ylpropan-1-ol |
a) i-B, ii-A, iii-D, iv-C
b) i-A, ii-C, iii-D, iv-B
c) i-D, ii-C, iii-B, iv-A
d) i-A, ii-B, iii-C, iv-D
Q.3 Complete the following sentence related with the mechanism of action of Procyclidine hydrochloride.
‘Procyclidine ………….. the central cholinergic receptors and ………..the cholinergic and dopaminergic activity in the basal ganglia.’
a) blocks, balances
b) stimulates, balances
c) balances, blocks
d) balances, stimulates
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Methacoline: Cholinergic antagonist
- Procyclidine: Muscarinic antagonist
- Homatropin: Cholinergic agonist
- Atropin: Muscarinic antagonist
a) FTFT
b) TTTF
c) TFTF
d) FFTT
Q.5 Maximum potency obtained when the distance between the ring substituted carbons in procyclidine hydrochloride is?
a) 1 carbon unit
b) 4 carbon unit
c) 2 carbon unit
d) 7 carbon unit
Q.6 The correct sequence for the steps for synthesis of drug Procyclidine hydrochloride from ‘cyclohexyl(phenyl) methanone’?
I. Treatment with pyrrolidine
II. Reaction with triphenylphosphine
III. Reaction with diborane
IV. Reaction with ethyl-2-bromoacetate
a) I – II
b) IV – III – II – I
c) I – III – II – IV
d) III – IV
Q.7 Side effect of drug procyclidine hydrochloride?
a) Allergic reactions
b) Dry mouth
c) Constipation
d) All of the above
ANSWERS
1-c
2-b
3-a
4-a
5-c
6-b
7-d
REFERENCES
[1] Schjelderup L, Harbitz O, Groth P, Aasen AJ. Syntheses of (S)-(+)-trihexyphenidyl hydrochloride and (S)-(+)-procyclidine hydrochloride, two anticholinergics, using (S)-(−)-3-cyclohexyl-3-hydroxy-3-phenylpropanoic acid as chiral synthon. Acta Chem. Scand.. 1987 May;41:356.