PROPANTHELINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
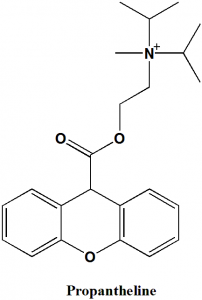
Propantheline
IUPAC nomenclature
Methyl-di(propan-2-yl)-[2-(9H-xanthene-9-carbonyloxy)ethyl]azanium
Classification
Propantheline is an acetylcholine antagonist. It is a muscarinic antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 368.5 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 152-162°C |
| 4 | Solubility | Very soluble in water |
| 5 | Presence of ring | Xanthenes |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
Propantheline reduces the effect of acetylcholine by blocking the receptors for acetylcholine on smooth muscles. It’s effects also includes the direct relaxation of the smooth muscles. [1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potent derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
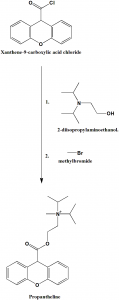
Method of synthesis
i. Xanthene-9-carboxylic acid chloride reacts with 2-diisopropylaminoethanol.
ii. Alkylation of the formed compound using methyl bromide gives propantheline.
Therapeutic Uses
Propantheline is used for:
- Reducing muscle spasms
- Treatment of peptic ulcers
Side Effects
Side effects of propantheline are:
- Constipation
- Nausea
- Vomiting
- Widened pupil
- Blurred vision
- Dizziness
- Decreased sweating
- Dry mouth
MCQ
Q.1 “Methyl-di(propan-2-yl)-[2-(9H-xanthene-9-carbonyloxy)ethyl]azanium” is the IUPAC nomenclature of which drug?
a) Trihexyphenidyl
b) Propantheline
c) Oxybutynin
d) Oxazepam
Q.2 Melting point of Propantheline is?
a) 152-162°C
b) 129-130°C
c) 205.5°C
d) 258.5°C
Q.3 Match the following with correct classifications of the drugs.
| i. Propantheline | A. Benzodiazepine sedative-hypnotic |
| ii. Oxazepam | B. Barbiturate |
| iii. Clozapine | C. Muscarinic antagonist |
| iv. Thiamylal | D. Benzpines |
a) i-A, ii-C, iii-D, iv-B
b) i-C, ii-A, iii-D, iv-B
c) i-A, ii-B, iii-D, iv-C
d) i-C, ii-B, iii-A, iv-D
Q.4 Mechanism of action of Propantheline is based on?
a) Blocking the Muscarinic acetylcholine receptors
b) Blocking the nicotinic acetylcholine receptors
c) Direct relaxation of smooth muscles
d) Both a) and c)
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug trihexyphenidyl
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide..
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
a) TFTF
b) FTFT
c) TTTT
d)FFTF
Q.6 Steps involved in the synthesis of Propantheline from xanthenes-9-carboxylic acid chloride in the correct sequence is?
I. Reaction with 2-diisopropylaminoethanol
II. Grignard’s reaction with cyclohexylmagnesium chloride
III. Alkylation using methylbromide
a) I – II – III
b) I – III
c) I – II
d) II – I – III
Q.7 The drug Propantheline is mainly used for?
a) Parkinson’s disease
b) Muscle spasm
c) Depression
d) Constipation
Participate in Free Online Test for GPAT
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-a
3-b
4-d
5-c
6-b
7-b