PSEUDOEPHEDRINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Pseudoephedrine
IUPAC nomenclature
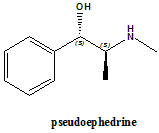
(S,S)-2-methylamino-1-phenylpropan-1-ol.
Classification
Pseudoephedrine is an indirect acting sympathomimetic amine drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 165.23 g/mol |
| 2 | Physical appearance | Present in solid form; crystals from ether. |
| 3 | Melting point | 119°C |
| 4 | Solubility | Sparingly soluble in water;freely soluble in alcohol and ether; very soluble in benzene |
| 5 | Octanol/water partition coefficient | 0.89 |
| 6 | Presence of ring | Benzene ring |
| 7 | Number of chiral centers | 2 |
Mechanism of Action
i. Pseudoephedrine produces agonism of α-adrenergic recepetors and less strongly to the ß-adrenergic receptors.
ii. This produces vasoconstriction.
iii. Further, pseudoephedrine also inhibits norepinephrine, dopamine, serotonin transportors, NF-kappa-B, NFAT and AP-1. [1]
Structure Activity Relationship
The SAR of Adrenergic agonist can be discussed as follows:
- Primary or secondary aliphatic amine separated by two carbons from a substituted benzene ring is essential for the high agonist activity.
- The hydroxyl substituted carbon must be in the R configuration for the maximal direct activity.
R1 substitution:
- When R1 is increased in size, activity of alpha receptors decreases and activity of the beta receptors increases
- Activity of both alpha and beta receptors is maximum when R1 is methyl group.
- Alpha agonist activity decreases when R1 is larger than methyl, and went negligible when R1 is isopropyl.
- Large lipophillic groups can afford compounds with alpha blocking activity.
- N-substituent provides selectivity for different receptors.
- Arylalkyl group can provide beta selectivity, increased cell penetration and increased lipophillicity for the longer duration of action.
R2 substitution:
- Ethyl group can eliminate the alpha activity of the drug.
- Erythrostero isomers have maximal activity.
- The additional methyl group makes the drug more selective for the alpha2
R3 substitution on the aromatic ring:
- 3’,4’-dihydroxy substituted benzene ring has poor oral activity.
- 3’, 5’-dihydroxy compounds are orally active.
- At least one of the groups is required which can form hydrogen bonds. And if only one group is present then it is preferred at 4’ position to retain the beta2
If phenyl group has no phenolic substituent then it may act directly or indirectly. [2]
Method of synthesis
i. Yeast strains like Candida utilis or Saccharomyces cerevisiae are added to the mixture of water, dextrose and pyruvate decarboxylase.
ii. Benzaldehyde is added to the vats afterwards.
iii. Yeast converts Dextrose into L-phenylacetylcarbinol
iv. Pseudoehedrine is produced from L-phenylacetylcarbinol via reductive amination.

Therapeutic Uses
Pseudophedrine is used:
- As a nasal decongestant
Side Effects
Side effects of pseudoephedrine are:
- Nausea
- Vomiting
- Trouble sleeping
- Headache
- Dizziness
- Nervousness
- Anxiety
- Confusion
- Restlessness
- Tremor
- Abdominal pain
- Difficulty in urination
- Allergic reactions
MCQs
Q.1 What can be the correct IUPAC nomenclature of Pseudoephedrine?
a) (±)-1-cyclohexyl-N-methylpropan-2-amine
b) 4-(2-aminopropyl)phenol
c) (S,S)-2-methylamino-1-phenylpropan-1-ol.
d) 3-[(1R,2S)-2-amino-1-hydroxypropyl]phenol
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Pseudoephedrine?
I. Primary or secondary aliphatic amine separated by two carbons from a substituted benzene ring is essential for the high agonist activity.
II. The hydroxyl substituted carbon must be in the R configuration for the maximal direct activity.
a) I
b) II
c) I, II
d) None
Q.3 The correct order for the synthesis of drug pseudoephedrine from dextrose can be?
I. Conversion to L-phenylacetylcarbinol by yeast.
II. Reductive amination
III. Addition of benzaldehyde
a) II – I – III
b) I – II – III
c) III – I – II
d) I – II – III
Q.4 Side effect of drug pseudoephedrine is?
a) Anxiety
b) Baldness
c) Increased blood calcium level
d) Decrease in blood count
Q.5 Match the following drugs with correct molecular weights-
| i. Pseudoephedrine | A. 155.28 gm/mol |
| ii. Hydroxyamphetamine | B. 165.23 gm/mol |
| iii. Metaraminol | C. 151.21 gm/mol |
| iv. Propylhexedrine | D. 167.2 gm/mol |
a) i-B, ii-C, iii-D, iv-A
b) i-D, ii-A, iii-B, iv-C
c) i-C, ii-B, iii-A, iv-D
d) i-A, ii-D, iii-C, iv-B
Q.6 An example of drug from class “Mixed Acting Sympathomimetics” is?
a) Dobutamine
b) Ritodrine
c) Epinephrine
d) Pseudoephedrine
Q.7 The type of ring system found in Pseudoephedrine is?
a) Purine ring system
b) Pyrimidine ring system
c) Benzene
d) No ring present
ANSWERS
1-c
2-d
3-c
4-a
5-a
6-d
7-c
REFERENCES
[1] Wee S, Ordway GA, Woolverton WL. Reinforcing effect of pseudoephedrine isomers and the mechanism of action. European journal of pharmacology. 2004 Jun 16;493(1-3):117-25. [2] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 348-352