Tonicity and its effects on red blood cells (RBCs)
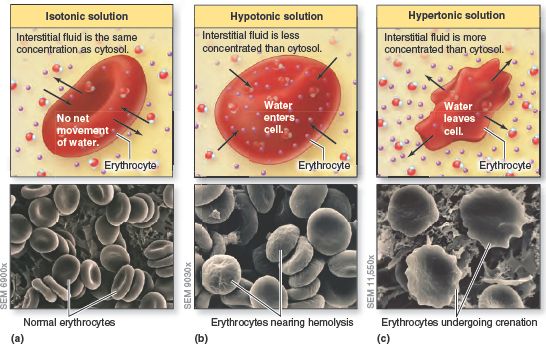
Normally, the osmotic pressure of the cytosol is the same as the osmotic pressure of the interstitial fluid outside cells. Because the osmotic pressure on both sides of the plasma membrane (which is selectively permeable) is the same, cell volume remains relatively constant. When body cells are placed in a solution having a different osmotic pressure than cytosol, however, the shape and volume of the cells change. As water moves by osmosis into or out of the cells, their volume increases or decreases.
A solution’s tonicity is a measure of the solution’s ability to change the volume of cells by altering their water content. Any solution in which a cell—for example, a red blood cell (RBC)—maintains its normal shape and volume is an isotonic solution. The concentrations of solutes that cannot cross the plasma membrane are the same on both sides of the membrane in this solution. For instance, a 0.9% NaCl solution (0.9 grams of sodium chloride in 100 mL of solution), called a normal (physiological) saline solution, is isotonic for RBCs. The RBC plasma membrane permits the water to move back and forth, but it behaves as though it is impermeable to Na and Cl , the solutes. (Any Na or Cl ions that enter the cell through channels or transporters are immediately moved back out by active transport or other means.) When RBCs are bathed in 0.9% NaCl, water molecules enter and exit at the same rate, allowing the RBCs to keep their normal shape and volume.
A different situation results if RBCs are placed in a hypotonic solution, a solution that has a lower concentration of solutes than the cytosol inside the RBCs. In this case, water molecules enter the cells faster than they leave, causing the RBCs to swell and eventually to burst. The rupture of RBCs in this manner is called hemolysis the rupture of other types of cells due to placement in a hypotonic solution is referred to simply as lysis. Pure water is very hypotonic and causes rapid hemolysis.
Image credit from www.pinterest.com and use for only educational purpose.
A hypertonic solution has a higher concentration of solutes than does the cytosol inside RBCs. One example of a hypertonic solution is a 2% NaCl solution. In such a solution, water molecules move out of the cells faster than they enter, causing the cells to shrink. Such shrinkage of cells is called crenation.
Find below more articles on transport across the cell membrane
Carrier Mediated Facilitated diffusion explained: Lecture and notes of Transport across the cell membrane : Click here
Membrane Protein : Click Here
Cell Membrane Permeability: Click Here
Channel Mediated Facilitated diffusion | Cellular Transport : Click Here