TOPOTECAN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
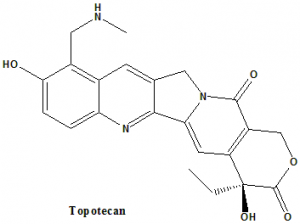
Topotecan
IUPAC nomenclature
(S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione monohydrochloride.
Classification
Toptecan falls under the category of camptothecin cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 421.4 g/mol |
| 2 | Appearance | Present in solid form |
| 3 | Melting point | Range between 213-218°C |
| 4 | Solubility | 2350 mg per liter in water |
| 5 | Octanol water partition coefficient | 0.8 |
| 6 | Presence of ring | Ring systems like quinoline and pyridine are present. |
Mechanism of Action
i. During the S phase of the DNA synthesis, topotecan binds with the topoisomerase I-DNA complex. This prevents the relegation of the DNA strands. Topotecan binds at the site of the DNA cleavage by intercalating between the upstream and downstream base pairs by mimicking a DNA base pair.
ii. The ternary complex so formed interferes with the moving replication fork.
iii. The lethal double-stranded breaks in the mammalian cells cannot be repaired by the cells and thus apoptosis takes place. [2]
Structural Activity Relationship
- The E ring in a lactone form is much more potent than the E-ring in the caboxylate form.
- For the activity of the drug, chiral center at position 20 is necessary.
- R-configuration is inactive form.
- Drug without the A and B rings shows less potency for the DNA synthesis inhibition.
- A and B rings are important for the activity of the drug. [3]
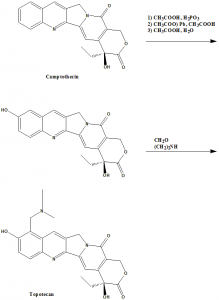
Methods of Synthesis
i. Reduction and selective oxidation of the quinoline moiety of the camptothecin.
ii. Introduction of the N,N-dimethylaminomethyl side chain.
Therapeutic Uses
- when other treatments fails during the cure of ovary cancer, this drug is prescribed.
- Topotecan is also used for the treatment of certain lung cancers like small cell lung cancer.
Side Effects
- Common side effects includes low blood counts, nausea,vomiting, hair loss and diarrhea.
- Some people may suffer from side effects like constipation, fatigue, fever, abdominal pain, bone pain, weakness, mouth sores, loss of appetite, skin reactions like rashes, shortness of breath, cough and headache.
MCQs
Q.1 Which term is NOT associated with the drug Topotecan?
a) Topotel
b) Duanocin
c) Oncotron
d) Both b) and c)
Q.2 Match the following with respect to the physical forms of drugs
| i. Tinidazole | A. White to pale yellow crystalline powder |
| ii. Metronidazole | B. present in solid form at room temperature |
| iii. Topotecan | C. colorless crystals |
| iv. Morphine | D. White crystalline solid |
a) i-A, ii-D, iii-C, iv-B
b) i-B, ii-A, iii-C, iv-D
c) i-C, ii-A, iii-B, iv-D
d) i-C, ii-D, iii-B, iv-A
Q.3 The drug Topotecan shows its action through-
a) Inhibiting the pyrimidine synthesis within the cell
b) Inhibiting the purine synthesis within the cell
c) Inducing radiosensitization in the cells
d) Binding with topoisomerase I-DNA complex
Q.4 The correct order for the synthesis of the drug Topotecan can be
I. Selective oxidation of the quinoline moiety.
II. Extraction of camptothecin from its natural source
III. Extraction of Epipodophyllotoxin from its natural source
IV. Introduction of N,N-dimethylaminomethyl side chain
a) III – I – IV
b) II – I – IV
c) III – IV – I
d) II –IV – I
Q.5 Predict the incorrect statements from the following with respect to the classification of the drugs.
I. Chlorambucil belongs to class Nitrogen mustard alkylating agent
II. Dacarbazine belongs to class Nitrosoureas
III. 6-Mercaptopurine belongs to pyrimidine antagonist
IV. Topotecan belongs to class Camptothecin analogues
a) All of the above
b) Only III & IV
c) Only II & III
d) Only I, II & III
Q.6 The correct sequence of True and False for the given statements with respect to the side effects of drug Topotecan is-
I. RBC and WBC counts may increase
II. RBC and WBC counts may decrease, platelet counts may increase
III. Hair loss is a common side effect of this drug
IV. Fatigue and fever may occur in the patient taking topotecan drug.
a) FTTT
b) FFTT
c) TFTT
d) FFFT
Q.7 Which amongst the following drugs is having highest number of ring system in its structure-
a) Etoposide
b) Tinidazol
c) Metronidazol
d) Topotecan
ANSWERS
1-d
2-c
3-d
4-b
5-c
6-b
7-a