TOREMIFENE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
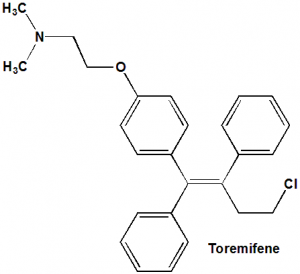
Toremifene
IUPAC nomenclature
2-[4-[(1Z)-4-Chloro-1,2-diphenyl-but-1-en-1-yl]phenoxy]-N,N-dimethylethanamine.
Classification
Toremifene is a selective estrogen receptor modulator. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 406 g/mol |
| 2 | Appearance | Present in the solid form |
| 3 | Melting point | 108-110°C |
| 4 | Solubility | 0.000409 mg/ ml in water |
| 5 | Octanol/water partition coefficient | 6.8 |
| 6 | Presence of ring | Aryl rings |
| 7 | Number of chiral centers | No chiral centers present. |
Mechanism of Action
- Toremifene binds to estrogen receptors and may exert antiestrogenic, estrogenic or both the properties depending upon the end point selected, targeted organ, gender and the duration of treatment.
- Due to its antiestrogenic properties, this drug helps in the treatment of breast cancer.
- The tumor growth is further checked by inducing apoptosis in the tumor cells.
Structural Activity Relationship
- Alkylaminoethane side chain is important for the antiestrogenic activity.
- Para hydroxylation of the phenyl ring on carbon 1 of butene can increase the potency of antiestrogen.
- The hydroxyl group is critical to locate the alkyl aminoethoxy side chain in the correct position in the steroid-binding site to block estrogen action.
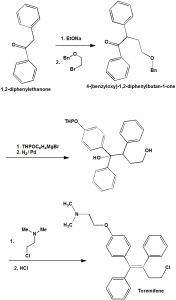
Method of synthesis
i. 1,2-diphenylethanone is reacted with EtONa and 1-((2-bromoethoxy)methyl)benzene to give 4-(benzyloxy)-1,2-diphenylbutan-1-one.
ii. The latter compound is then reacted with THPOC6H4MgBr, and thereafter it is reduced.
iii. In the next step, the formed compound is reacted with 2-chloro-N,N-dimethylethanamine.
iv. Treatment with HCl gives toremifene.
Therapeutic Uses
The drug used for the treatment of metastatic breast cancer in the female patients who are postmenopausal and the tumors are estrogen receptor positive.
Side Effects
- Common side effects of Toremifene include vaginal discharge, sweating, nausea, vomiting, poor appetite and hot
- Less common side effects are depression, insomnia, weight gain, swelling, dizziness, headache and pain.
MCQs
Q.1 Which term is associated with the drug Toremifene?
a) Fareston
b) Shantinib
c) Oncocarbin
d) Honvan
Q.2 Match the following with respect to the approximate molecular weights of drugs
| i. Toremifene | A. 296 gm/mol |
| ii. Ethinylestradiol | B. 406gm /mol |
| iii. Tamoxifen | C. 360 gm/mol |
| iv. Prednisolone | D. 371.5 gm/mol |
a) i-B, ii-A, iii-C, iv-D
b) i-A, ii-B, iii-D, iv-C
c) i-A, ii-B, iii-C, iv-D
d) i-B, ii-A, iii-D, iv-C
Q.3 The drug Toremifene shows its action through
a) Binds with estrogen receptors and shows estrogenic properties
b) Binds with estrogen receptors and shows antiestrogenic properties
c) Binds with estrogen receptor and shows either estrogenic, antiestrogenic or both the properties.
d) None of the above
Q.4 The correct order for the synthesis of the drug Toremifene is?
I. Reaction with 2-chloro-N,N-dimethylethanamine
II. Reaction with THPOC6H4MgBr and reduction.
III. 1,2-diphenylethanone is reacted with EtONa and 1-((2-bromoethoxy)methyl)
IV. Reaction with HCl.
a) II – III – I – IV
b) IV – III – I – II
c) I – II – IV – III
d) III – II – I – IV
Q.5 Predict the incorrect statements from the following with respect to the classification of the drugs-
I. Prednisolone is a glucocorticoid.
II. Fosfestrol is a selective estrogen down regulator.
III. Torenifene is a selective estrogen modulator.
IV. Nafarelin is a GnRH analogue.
a) II & IV
b) II
c) III & IV
d) I & II
Q.6 The correct sequence of True and False for the given statements with respect to the side effects of drug Tornifene is?
I. Vaginal discharge is a common side effect.
II. Depression is a less common side effect.
III. Increase in appetite may occur in patients taking the drug.
IV. Insomia is a less common side effect.
a) TFTF
b) TTFT
c) FFTF
d) FTFT
Q.7 Which amongst the following drugs has the highest melting point?
a) Toremifene
b) Prednisolone
c) Tamoxifen
d) Ethinylestradiol
ANSWERS
1-a
2-d
3-c
4-d
5-b
6-b
7-b
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Kangas L. Review of the pharmacological properties of toremifene. Journal of steroid biochemistry. 1990 Jun 22;36(3):191-5.