TRIPTORELIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
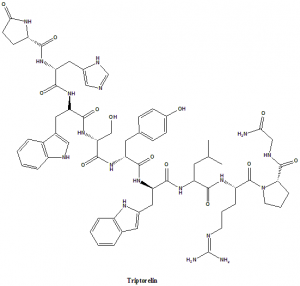
Triptorelin
IUPAC nomenclature
5-oxo-D-prolyl-L-histidyl-Ltryptophyl-L-seryl-Ltyrosyl-3-(1H-indol-2-yl)-L-alanylleucyl-L-arginyl-L-prolylglycinamide.
Classification
Triptorelin is a GnRH analogue. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 1311.4 g/mol |
| 2 | Physical appearance | White to off white powder.
Present in salt form as triptorelin pamoate |
| 3 | Melting point | More than 160°C |
| 4 | Solubility | Freely soluble in acetic acid; soluble in water. |
| 5 | Presence of ring | Ring structures present |
| 6 | Number of chiral centers | 9 |
Mechanism of Action
- It is a synthetic agonist analogue of GnRH (gonadotropin releasing hormone)
- 13 fold higher releasing activity for luteinizing hormone.
- 21 fold higher releasing activity for FSH hormone.
Structure Activity Relationship
- When Gly 6 is replaced by certain D-amino acids, or if there is change in the G-terminus, they became less susceptible to proteolytic enzymes, thus, provides longer lasting actions.
- Half life of the drug can be increased by substituting hydrophobic D-amino acids at the glycine near endopeptidase cleavage. [2]
Method of synthesis
Rink amide AM resins or Rink amide MBHA resins are taken as starting chemicals. According to the solid phase synthesis, amino acids with protective groups are sequentially connected to give protective decapeptide resins. Crude product is obtained through sequential removing of Fmoc-protective groups and removal of the side chain protective groups and cutting peptides.
Crude products are separated and purified by C18 column and freeze-dried.
Therapeutic Uses
The drug used for the treatment of:
- Advanced prostate cancer
Side Effects
Common side effects of triptorelin are inability of sustaining erection, decreased libido and hot flashes.
Less common side effects are sweating , depression, swelling of breast, loss of strength, edema, increased cholesterol level, discomfort at injection site, hypertension, muscle and bone pain, breast pain and urinary retention.
MCQs
Q.1 ‘Trelstar’ and ‘triptodur’ are the trade names of which drug??
a) Nafarelin
b) Triptorelin
c) Anaastrozole
d) Letrozole
Q.2 Predict the correct statement related to the therapeutic uses of drug triptorelin.
a) It is used for the treatment of Prostate cancer.
b) It is used as a beta blocker.
c) It is given during hypertension.
d) It is given during hypotension
Q.3 The correct statement related to the solubility of Triptorelin drug is?
a) Freely soluble in acetic acid; soluble in water
b) Soluble in acetic acid; sparingly soluble in water
c) Soluble in acetic acid; freely soluble in water
d) Soluble in acetic acid; insoluble in water
Q.4 Which amongst the following drugs is a synthetic agonist analogue of GnRH?
a) Triptorelin
b) Methotrexate
c) Cytarabine
d) Vinblastine
Q.5 Triptorelin drug belongs to which class?
a) Antiandrogens
b) Aromatase inhibitors
c) GnRH analogue
d) Antibiotics
Q.6 Which of the following is NOT a side effect of Triptorelin?
a) Decreased libido
b) Depression
c) Increased cholesterol level
d) Hypotension
Q.7 Which amongst the following drugs is having least number of ring system in its structure-
a) Nafarelin
b) Phenylephrin
c) Triptorelin
d) Finasteride
ANSWERS
1-b
2-a
3-a
4-a
5-c
6-d
7-b
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 221.