UMIFENOVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses in Influenza
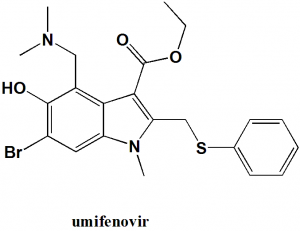
Umifenovir
IUPAC nomenclature
Ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-meth yl-2-[(phenylsulfanyl)methyl]-1H-indole-3-carboxylate.
Classification
Umifenovir belongs to class fusion inhibitors.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 477.74 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 133-137°C |
| 4 | Solubility | Water solubility is 0.00678 mg/ml |
| 5 | Presence of ring | Indole, benzene |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
The drug acts on aromatic residues within the glycoprotein which are important for cellular recognition and fusion. This interfere with clathrin-mediated exocytosis and intracellular trafficking, directly at the plasma membrane itself. This stabilizes the plasma membrane and prevent the entry of virus. [1]
Structure Activity Relationship
- Increasing the size of the group at indoleposition 4 from a dimethylamino group to a piperazine do not dramaticallyimprove binding to H3.
- Increasing the size of the aromatic ring at position 2 does not generate improved interactions, nor did the addition of a meta-NH2 with respect to the thiol.
- Binding affinity increases by eightfold when the dimethylamino group at indole position 4 on Arbidol is removed for H3 HA.
- By attaching a hydroxy group in the meta-position, it would be possible to increase the binding by displacing an ordered water molecule the binding pocket next to Arbidol.
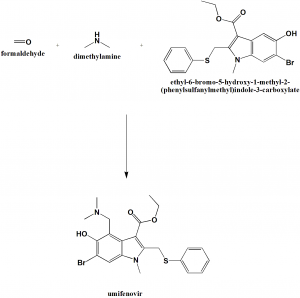
Method of synthesis
Formaldehyde, dimethylamine and ethyl-6-bromo-5-hydroxy-1-methyl-2-(phenylsulfanylmethyl)indole-3-carboxylate to get Umifenovir.
Therapeutic Uses
Umifenovir is used for:
- Treatment of symptoms of influenza virus.
- Treatment of hepatitis C
- Investigation drug for the treatment for COVID-10/SARS-CoV-2 Outbreak
Side Effects
Side effects of Umifenovir are:
- Sensitization of the drug
- Allergic reactions
MCQ
Q.1 Choose the correct statements related to the physicochemical properties of drug umifenovir-
I. Molecular weight is 477.74 gm/mol
II. Present in solid form
III. It is very soluble in water
IV. Melting point is 273K
a) I, II
b) I, III
c) III, IV
d) I, III, IV
Q.2 Match the following of the drugs with their correct Trade names.
| i. Umifenovir | A. Topotel |
| ii. Daunorubicin | B. Arbidol |
| iii. Topotecan | C. Dacmozen |
| iv. Dactinomycin | D. Myeleran |
a) i-B, ii-C, iii-D, iv-A
b) i-B, ii-C, iii-A, iv-D
c) i-C, ii-A, iii-D, iv-B
d) i-A, ii-D, iii-B, iv-C
Q.3 Umifenovir do not let the virus to?
a) Use the host machinery
b) Infect other cells
c) Grow in size
d) Synthesize DNA
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Umifenovir: Neuraminidase inhibitor analogue
- Cidofovir: Nonnucleoside reverse transcriptase inhibitor
- Ribavirin: Conventional nucleoside analogue
- Tipranavir: HIV protease inhibitor
a) TTTT
b) TTTF
c) FTTT
d) TFTT
Q.5 When the dimethylamino group at indole position-4 arbidol is removed?
a) Biding affinity increases
b) Activity of drug increases
c) Biovailability increases
d) Activity decreases
Q.6 Type/s of ring present in the structure of Umifenovir?
I. Cyclopentyle
II. Indole
III. Benzene
a) II, III
b) I, II
c) I
d) I, II, III
Q.7 Side effect of drug Umifenovir?
a) Allergic reactions
b) Hallucinations
c) Confusion
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-b
3-b
4-d
5-a
6-a
7-a