Vincristine (Oncovin) Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Vincristine (Oncovin)
IUPAC nomenclature
(3aR,3a1R,4R,5S,5aR,10bR)-Methyl 4-acetoxy-3a-ethyl-9-((5S,7S,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-2,4,5,6,7,8,9,10-octahydro-1H-3,7-methano[1]azacycloundecino[5,4-b]indol-9-yl)-6-formyl-5-hydroxy-8-methoxy-3a,3a1,4,5,5a,6,11,12-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate.
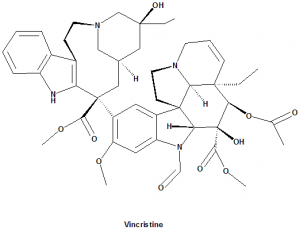
Classification
Vincristine falls under the category of Vinca alkaloids cytotoxic drugs.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 825 g/mol |
| 2 | Appearance | White crystalline solid |
| 3 | Melting point | 220 °C |
| 4 | Solubility | 2.27 mg per liter in water |
| 5 | Octanol water partition coefficient | 2.82 |
| 6 | Presence of ring | Indole, piperidine and quinoline ring systems present. |
Mechanism of Action
- Vincristine can interfere with amino acids, cAMP, nucleic acid biosynthesis, lipid biosynthesis, glutathione metabolism and calmodulin dependent Calcium transport ATPase activity.
- But the primary activity of this drug is through inhibition of the mitosis at the metaphase stage of the cell by interaction with the tubuline protein.[1]
Structural Activity Relationship
- Aryl or heteroaryl compounds decreases the activity of the drug.
- Strongly polar or charged groups also decreases the activity of the drug.
- Decreasing in the polarity of the substituent produces more active analogues of the drug.
- Decreasing the charge on the substituent also increases the activity of the analogues of the drug.
- The activity of the drug can be restores by the introduction of the lipophillic linker such as thioether.
- Small and uncharged molecules increase the activity and shows best results. [2]
Methods of Synthesis
i. Alkaloids present in the Catharanthus roseus plant are extracted and separated into alkaloid tartrate with the help of benzene.
ii. Vincristine is separated through the process of chromatography on aluminium oxide deactivated with acetic acid.
Total synthesis method
i. The the chloroindolenine was treated with trifluoroacetic acid to give coupling product.
ii. Subsequent selective methanolysis of the trifluoroacetate to give tertiary alcohol.
iii. Tertiary alcohol is then treated with 2-mercaptoethanol and 1,8-diazabicyclo[5.4.0]undec-7-ene in acetone to remove 2-nitrobenzenesulfonyl group to produce the corresponding secondary amine.
iv. The piperidine ring of the upper part is then constructed by intramolecular SN2 reaction to furnish demethylvinblastine.
v. At last, formylation of N1 position gives (+)-vincristine.

Therapeutic Uses
- Acute leukemia
- Hodgkin’s and non-Hodgkin’s lymphoma
- Brain tumors
- Thyroid cancers
- Chronic leukemia
- Multiple myeloma
- Neuroblastoma
- Rhabdomyosarcoma
- Ewing’s sarcoma
- Some blood disorders
Side Effects
- Hair loss is a common side effect
- Less common side effects include constipation, low blood count, peripheral neuropathy, taste changes, loss of appetite, diarrhea, nausea and vomiting, weight loss and mouth sores.
MCQs
Q.1The terms ‘Leurocristin’ and ‘Oncovin’ are associated with which drug?
a) Triptorelin
b) Thiotepa
c) Vincristine
d) Topotecan
Q.2 The correct statement for the SAR of the drug Vincristine is-
a) Aryl or heteroaryl compounds increases the activity of the drug.
b) Strongly polar or charged groups also increases the activity of the drug.
c) Decreasing the charge on the substituent also increases the activity of the analogues of the drug.
d) Small and uncharged molecules increase the activity and shows best results.
Q.3 Which amongst the following are the correct side effects of the drug?
I. Hair loss
II. Increase in blood count
III. Abdominal cramps
IV. Weight gain
a) I, II & III
b) I & III
c) I, II & IV
d) I, III & IV
Q.4 Drug Vincristine can be extracted from which plant?
a) Cantharus roseus
b) Emblica officinalis
c) Saraca asoka
d) Withania somnifera
Q.5 Which pairs of drug and its classification are true
| I. | Vincristine | Vinca alkaloids |
| II. | Etopside | Vinca alkaloids |
| III. | Carmustine | Nitrosoureas |
| IV. | Cytarabine | Nitrosoureas |
a) I, II, III & IV
b) I, II & IV
c) I & III
d) I, III & IV
Q.6 Correct physical form in which the drug Vincristine is found?
a) White granular powder
b) White crystalline solids
c) Red to yellow powder form
d) None of the above
Q.7 Match the following with respect to ring systems of the drug
| i. Tinidazole | A. No ring present |
| ii. Mechlorethamine | B. Oxazophosphorine ring |
| iii. Vincristine | C. Imidazole ring |
| iv. Cyclophosphamide | D. Indole ring |
i-C, ii-A, iii-D, iv-B
i-B, ii-C, iii-A, iv-D
i-D, ii-B, iii-A, iv-C
i-A, ii-D, iii-B, iv-C
ANSWERS
1-c
2-d
3-b
4-a
5-c
6-b
7-a
REFERENCES
[1] Tsutsui T, Suzuki N, Maizumi H, Barrett JC. Vincristine sulfate-induced cell transformation, mitotic inhibition and aneuploidy in cultured Syrian hamster embryo cells. Carcinogenesis. 1986 Jan 1;7(1):131-5. [2] Voss ME, Ralph JM, Xie D, Manning DD, Chen X, Frank AJ, Leyhane AJ, Liu L, Stevens JM, Budde C, Surman MD. Synthesis and SAR of vinca alkaloid analogues. Bioorganic & medicinal chemistry letters. 2009 Feb 15;19(4):1245-9.