ZANAMIVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Zanamivir
IUPAC nomenclature
(2R,3R,4S)-4-guanidino-3-(prop-1-en-2-ylamino)-2-((1R,2R)-1,2,3-trihydroxypropyl)-3,4-dihydro-2H-pyran-6-carboxylic acid.
Classification
Zanamivir is a Neuraminidase inhibitor analogue.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 332.31 g/mol |
| 2 | Physical appearance | White to off-white powder |
| 3 | Melting point | 256°C |
| 4 | Solubility | 18 g/L in water |
| 5 | Octanol/water partition coefficient | -3 |
| 6 | Presence of ring | Pyrane |
| 7 | Number of chiral centers | 5 |
Mechanism of Action
- Drug binds with neuraminidase protein and inhibits it which unable the virus to escape from the host cell and infect other cells.
Structure Activity Relationship
- Zanamivir sulfonate exhibit stronger binding to avian influenza neuraminidase H5N1 than their carboxylate and phosphate analogues.
- Alkoxyl ester derivatives have better bioavailability when administered orally.
- C-4 modified drug having different alkyl chains are most efficient.
- The C-4 derivatization of drug with thiocarbamates, α-amino acids or cyclic secondary amines led to decreased inhibitory activities.
- L-aspargine bearing analogues show best results. [1]
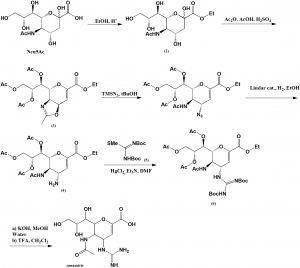
Method of synthesis
i. Neu5Ac is converted into ethyl ester (1).
ii. Treatment with acetic anhydride in acetic acid containing a catalytic amount of sulfuric acid to give (2).
iii. Ring opening reaction with trimethylsilyl azide.
iv. (4) undergoes hydrogenation and converts into guanidine derivative (6) by treatment with (5) using HgCl2 as the promoter
v. Zanamivir is isolated after saponification and removal of tert-butoxycarbonyl using TFA. [1]
Therapeutic Uses
Zanamivir is used for:
- Treatment of symptoms of influenza virus.
- Swine flu treatment
Side Effects
Side effects of Zanamivir are:
- Dizziness
- Increased cough
- Breathing problems
MCQ
Q.1 Match the following with correct SAR of the drug zanamivir-
| i. C-4 modified drug having different alkyl chains | A. decreased inhibitory effects |
| ii. Alkoxy ester derivatives | B. Better bioavailability when given orally. |
| iii. Sulfonates | C. stronger binding with influenza |
| iv.C-4 derivaization with thiocarbamates | D. most efficient |
a) i-A, ii-C, iii-B, iv-D
b) i-C, ii-A, iii-B, iv-D
c) i-B, ii-A, iii-C, iv-D
d) i-D, ii-B, iii-C, iv-A
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Zanamivir: 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-1,2,4-triazole-3-carboxamide.
- Favipiravir: 7-chloro-5-(2-fluorophenyl)-1-(2,2,2-trifluoroethyl)-3H-1,4-benzodiazepine-2-thione
- Lopinavir: 22,23-dihydroavermectin B1a + 22,23-dihydroavermectin B1b
- Benztropin: (RS)-2-[{4-[(7-chloroquinolin-4-yl)amino]pentyl}(ethyl)amino]ethanol
a) TFTF
b) TTFF
c) FFFF
d) TFTT
Q.3 Physical appearance of zanamivir drug is?
a) White to off white powder
b) Pale yellow powder
c) Yellow liquid
d) Transparent oil
Q.4 Zanamivir binds with?
a) Neuraminidase protein
b) α-receptors
c) Nicotinic receptor
d) None of the above
Q.5 Which amongst the following is a therapeutic use of drug Zanamivir?
a) Treatment of hepatitis C
b) Treatment of anxiety
c) Treatment of influenza
d) Treatment of HIV
Q.6 Which of the following drug and their classification are correct?
I. Acyclovir: Nonbezodiazepine sedative-hypnotic
II. Cidofivir: Synthetic guanosin neucleoside
III. Boceprivir: Non nucleoside analogue
IV. Amrenavir: HIV protease inhibitor
a) II, III
b) III, I
c) II, IV
d) III, IV
Q.7 Octanol/water partition coefficient of zanamivir is?
a) -3
b) 2
c) 1.85
d) -2.0
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-d
2-b
3-a
4-a
5-c
6-d
7-a
REFERENCES
[1] Laborda P, Wang SY, Voglmeir J. Influenza neuraminidase inhibitors: synthetic approaches, derivatives and biological activity. Molecules. 2016 Nov;21(11):1513.