PACLITAXEL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Paclitaxel
IUPAC nomenclature
(2α,4α,5β,7β,10β,13α)-4,10-Bis(acetyloxy)-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate.
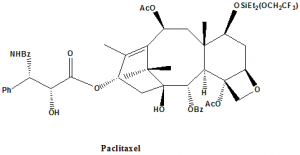
Classification
Paclitaxel falls under the category of taxanes.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 853.9 g/mol |
| 2 | Appearance | Fine white powder |
| 3 | Melting point | 213-216 °C |
| 4 | Solubility | Insoluble in water |
| 5 | Octanol water partition coefficient | 3 |
| 6 | Presence of ring | Taxane ring |
Mechanism of Action
i. Paclitaxl got bound with beta-subunit of tubuline protein.
ii. This leads to the locking of the microtubules at their places and cell cannot use the microtubules in an effective way.
iii. This negatively affects the cell’s transportation mechanism and also inhibit the mitosis of the cell.
iv. Further, paclitaxel binds with stopping protein Bcl-2 and induces apoptosis in the cell. [1]
Structural Activity Relationship
- Phenyl moieties at C-3’N, C-3’ and C-2 positions are not necessary for the cytotoxicity and the anti-mitotic activity of the drug.
- Replacing the phenyl groups with t-butyl groups can increase the activity of the drug.
- Replacing the phenyl groups with t-butyl groups can increase the potency to induce apoptosis in the cell. It can also increase the bioavailability of the drug. therefore, this type of modification was considered as the best modification of the drug paclitaxel.
- Modification at the C-3 position of the C-2 benzoate with CN, N3, MeO, and Cl increases the anticancer activity against P-388 cell line. [2]
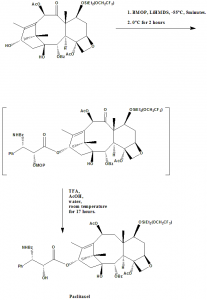
Methods of Synthesis
i. 7-OSiEt2(OCH2CF3)-baccatin-III is coupled with ((3R-cis)-1-Benzoyl-3-(1-methoxy-1-methylethoxy)-4-phen-yl-2-azetidinone) (BMOP) in anhydrous THF at -55°C to form an MOP intermediate.
ii. The formed MOP intermediate is then hydrolyzed with TFA/AcOH/Water for 17 hours.
iii. Paclitaxel can be obtained finally through chromatography.[3]
Therapeutic Uses
Paclitaxel is used for the treatments of:
- Breast cancers
- Ovarian cancers
- Lung cancers
- Bladder cancers
- Prostate cancers
- Melanomas
- Esophageal cancers
- Other solid tumor cancers
- Kaposi’s sarcoma
Side Effects
- Common side effects includes low blood counts, hair loss, arthralgias, myalgias, periphery neuropathy, nausea, vomiting, diarrhea, mouth sores and hypersensitivity reactions.
- Some people may suffer from side effects like edema, increase in blood test liver function, low blood pressure, darkening of the skin and changes in the color of the nails.
MCQs
Q.1 ‘Taxol A’ is the synonym of which drug?
a) Topotecan
b) Paclitaxel
c) Vinblastine
d) Fludarabine
Q.2 Predict the incorrect statement related to the therapeutic uses of drug Paclitaxel-
a) it is used for the treatment of Breast cancer
b) Used for treatment of myelomas
c) It is used for the treatment of low blood pressure
d) It is used for the treatment of prostate cancer
Q.3 Match the following with respect to the SAR of drug Paclitaxel
| i. Phenyl group at C-2 position | A. Not essential for the toxicity of the drug. |
| ii. Modification at the C-3 position of the C-2 benzoate with CN | B. Essential for the toxicity of the drug. |
| C. Decreases the activity of the drug. | |
| D. Increases the anticancer activity of the drug. |
a) i-A, ii-D
b) i-A, ii-C
c) i-B, ii-D
d) i-B, ii-C
Q.4 Which amongst the following drugs shows its effect through binding with tubuline proteins?
a) Nafrelin
b) Morphine
c) Letrozole
d) Paclitaxel
Q.5 Paclitaxel drug belongs to which class?
a) Progestins
b) Antiandrogens
c) Triazines
d) Taxanes
Q.6 Which of the following is not a side effect of Paclitaxel?
a) High blood pressure
b) Low blood count
c) Hair loss
d) Increase in the blood liver function
Q.7 Which amongst the following drugs is having least number of ring system in its structure-
a) Paclitaxel
b) 6-Mercaptopurine
c) 6-Thioguanine
d) Lomustine
ANSWERS
1-b
2-c
3-a
4-d
5-d
6-a
7-c