PERAMIVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
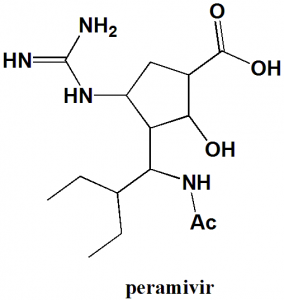
Peramivir
IUPAC nomenclature
(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylbutyl]-4-(carbamimidoylamino)-2-hydroxycyclopentanecarboxylic acid.
Classification
Peramivir is a Neuraminidase inhibitor analogue.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 328.41 g/mol |
| 2 | Physical appearance | White powder |
| 3 | Melting point | 257-262°C |
| 4 | Solubility | Sparingly soluble in deionised water |
| 5 | Presence of ring | Cyclopentyl |
| 6 | Number of chiral centers | 5 |
Mechanism of Action
- Drug binds with neuraminidase protein and inhibits it which unable the virus to escape from the host cell and infect other cells.
Structure Activity Relationship
- Peramivir sulfonate exhibit stronger binding to avian influenza neuraminidase H5N1 than their carboxylate and phosphate analogues.
- Alkoxyl ester derivatives have better bioavailability when administered orally.
- C-4 modified drug having different alkyl chains are most efficient.
- The C-4 derivatization of drug with thiocarbamates, α-amino acids or cyclic secondary amines led to decreased inhibitory activities.
- L-aspargine bearing analogues show best results. [1]
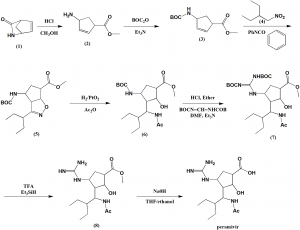
Method of synthesis
i. Lactan is dissolved I alcoholic solution, esterification by ring opening under HCl and acid catalysis.
ii. Compound formed is reacted with hydrochloride and tert-butyl dicarbonate.
iii. Reaction with oxammonium hydrochloride reactant salt in alcohol-carbonate system.
iv. Cycloaddition reaction takes place under base catalysis.
v. Hydrogenation in alcoholic solution to generate intermediate compound.
vi. Taking off BOC protect hydrochloride
vii. Two guanidine radicals are put in the reaction vessel, system made of alcohol, organic solvent, aqueous sodium hydroxide solution.
viii. Taking off BOC, product is obtained under organic acid.
Therapeutic Uses
Peramivir is used for:
- Treatment of symptoms of influenza virus.
- In Pre-clinical Stages it is used for COIVD-19 for investigation purpose
Side Effects
Side effects of Peramivir are:
- Mood changes
- Hallucinations
- Confusion
MCQ
Q.1 Choose the correct statements related to the physicochemical properties of drug peramivir-
I. Molecular weight is 328.41 gm/mol
II. It is white powder in appearance
III. It is very soluble in water
IV. Melting point is 273K
a) I, II
b) I, III
c) III, IV
d) I, III, IV
Q.2 Match the following of the drugs with their correct Trade names.
| i. Peramivir | A. Alkeran |
| ii. Melphalan | B. Endoxan |
| iii. Busulphan | C. Rapivab |
| iv. Cyclophosphamide | D. Myeleran |
a) i-B, ii-C, iii-D, iv-A
b) i-B, ii-C, iii-A, iv-D
c) i-C, ii-A, iii-D, iv-B
d) i-A, ii-D, iii-B, iv-C
Q.3 Peramivir do not let the virus to?
a) Use the host machinery
b) Infect other cells
c) Grow in size
d) Synthesize DNA
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Peramivir: Neuraminidase inhibitor analogue
- Delavirdine: Nonnucleoside reverse transcriptase inhibitor
- Ribavirin: Conventional nucleoside analogue
- Tipranavir: HIV protease inhibitor
a) TTTT
b) TTTF
c) FTTT
d) TFTT
Q.5 Correct statement related with the SAR of Peramivir is?
a) Alkoxy derivatives have better bioavailability when administered orally
b) Alkoxy derivatives have better activity
c) Alkoxy derivatives have lower half life
d) Alkoxy derivatives have lower activity
Q.6 Type/s of ring present in the structure of peramivir?
I. Cyclopentyle
II. Imidazole
III. Benzene
a) II, III
b) I, II
c) I
d) I, II, III
Q.7 Side effect of drug peramivir?
a) Mood changes
b) Hallucinations
c) Confusion
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-c
3-b
4-a
5-a
6-c
7-d
REFERENCES
[1] Laborda P, Wang SY, Voglmeir J. Influenza neuraminidase inhibitors: synthetic approaches, derivatives and biological activity. Molecules. 2016 Nov;21(11):1513.