MEPHOBARBITAL Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
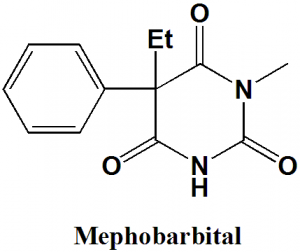
Mephobarbital
IUPAC nomenclature
5-Phenyl-5-ethyl-1-methylbarbituric acid
Classification
Mephobarbital is a barbiturate sedative-hypnotic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 246.26g/mol |
| 2 | Physical appearance | White crystals |
| 3 | Melting point | 176°C |
| 4 | Octanol/water partition coefficient | 1.84 |
| 5 | Solubility | Slightly soluble in water |
| 6 | Presence of ring | Pyridine, phenyl |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
i. Drug binds with different binding sites associated with chloride ionopore at the GABAA
ii. This results in increase in the duration of time for the opening of the chloride ionopore.
iii. As a result, the post synaptic inhibitory effect of GABA in the thalamus is prolonged.
Structure Activity Relationship
- Tri-keto form is most stable in aqueous solution.
- 4,6-dialcoholic tautomeric forms are least stable in aqueous solution.
- 5,5-disubstituted barbituric acid is the prime requirement for the barbiturates to be sedative hypnotics.
- Esterification of either of the 1,3-diazine nitrogens decreases hypnotic activity.
- Substitution of either of the 1,3-diazine nitrogens with aliphatic carbons retains the anticonvulsive properties.
- Esterification of the 5th-position substituents yields agents with analgesic activity but with weak hypnotic properties.
- Introduction of the polar functional group at the 5th– position yields compounds which are fully devoid of sedative-hypnotic or anticonvulsive activity.
- As the number of carbons at R2 carbon increases, the lipophillicity of the drug increases.
- Modification of the 2nd-position oxygen of the barbiturate backbone with sulfur atom yields thiobarbiturate derivatives with increased lipophillicity, shorter duration of action, faster time of onset compared to oxy-derivative. [1]
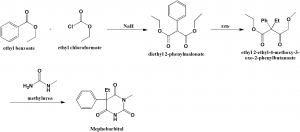
Method of synthesis
i. Ethylbenzoate reacts with ethyl chloroformate in presence of sodium hydride to give diethyl 2-phenylmalonate.
ii. diethyl 2-phenylmalonate undergoes alkylation to produce ethyl 2-ethyl-4-methoxy-3-oxo-2-phenylbutanoate.
iii. On condensation with methylurea, mephobarbital is yielded. [2]
Therapeutic Uses
Mephobarbital is used:
- As an anticonvulsive drug
- As a sedative
- As an anxiolytic.
Side Effects
Side effects of Mephobarbital are:
- Insomnia
- Dizziness
- Drowsiness
- Loss of coordination
- Nausea
- Vomiting
- Constipation
- Restlessness
- Headache
- Loss of appetite
- Pale skin
- Shallow breathing
MCQ
Q.1 “5-Phenyl-5-ethyl-1-methylbarbituric acid” is the IUPAC nomenclature of which drug?
a) Mephobarbital
b) Acyclovir
c) Zidovudin
d) Abacavir
Q.2 Correct statement related to the solubility of mephobarbital in water is?
a) Very soluble
b) Soluble
c) Slightly soluble
d) Practically insoluble
Q.3 Match the following with correct classifications of the drugs.
| i. Mephobarbital | A. Muscarinic agonist |
| ii. Methacholine | B. Nicotinic antagonist |
| iii. Atropine | C. Barbiturate sedative-hypnotic |
| iv. Metocurine | D. Muscarinic antagonist |
a) i-C, ii-A, iii-D, iv-B
b) i-B, ii-A, iii-D, iv-C
c) i-A, ii-C, iii-B, iv-D
d) i-D, ii-A, iii-B, iv-C
Q.4 Correct steps for the mechanism of action of the drug Mephobarbital
I. Binding of drug with GABA molecule
II. Binding of Drug with GABAA receptor
III. Increase in GABA affinity
IV. Increase in opening duration of the chloride channel
a) IV – II – I
b) I – III – IV
c) I – II – IV
d) II – III – IV
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug Mephobarbital.
- Esterification of the 5th-position substituents yields agents with analgesic activity but with weak hypnotic properties.
- Introduction of the nonpolar functional group at the 5th– position yields compounds which are fully devoid of sedative-hypnotic or anticonvulsive activity.
- As the number of carbons at R2 carbon decreases, the lipophillicity of the drug increases.
- Modification of the 2nd-position oxygen of the barbiturate backbone with sulfur atom yields thiobarbiturate derivatives with increased lipophillicity, shorter duration of action, faster time of onset compared to oxy-derivative.
a) TFFT
b) FTTF
c) FFTF
d) TTFT
Q.6 Starting materials for the synthesis of mephobarbital is/are?
a) Ethylbenzoate
b) Ethylchloroformate
c) Both a) and b)
d) None of the above
Q.7 The drug Mephobarbital is mainly used for?
a) As an anticonvulsive drug
b) For the treatment of HIV
c) For Treatment of Goiter
d) All of the above
Participate in Free Online Test for GPAT
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-c
3-a
4-d
5-a
6-c
7-a
REFERENCES
[1] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 490-91 [2] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10..