PHENSUXIMIDE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
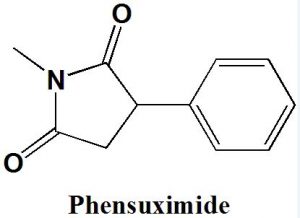
Phensuximide
IUPAC nomenclature
1-methyl-3-phenyl-pyrrolidine-2,5-dione
Classification
Phensuximide is a succinimide anticonvulsant.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 189.21 g/mol |
| 2 | Physical appearance | Present in solid form |
| 3 | Melting point | 72°C |
| 4 | Solubility | 7020 mg/L |
| 5 | Octanol/water partition coefficient | 0.7 |
| 6 | Presence of ring | Pyrrolidine, phenyl |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
- It inhibits neuronal systems that are important for generation of three per second rhythm.
- It inhibits depolarization-induced accumulation of cyclic adenosine monophosphate and GMP in brain tissues.
Structure Activity Relationship
SAR of succinimides can be discussed as follows:
- Phenyl substitution makes them active against electrically induced convulsion.
- N-methylation decreases activity against electroshock seizures.
- N-methylation also increases the activity against chemically induced convulsions.
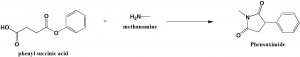
Method of synthesis
Phensuximide can be synthesized by the reaction of phenylsuccinic acid or its anhydride with methylamine. [1]
Therapeutic Uses
Phensuximide is used for:
- The treatment of epilepsy
Side Effects
Side effects of Phensuximide are:
- Dizziness
- Drowsiness
MCQs
Q.1 “1-methyl-3-phenyl-pyrrolidine-2,5-dione” is the IUPAC nomenclature of which drug?
a) Phensuximide
b) Halothane
c) Dutasteride
d) Fulvestrant
Q.2 Correct melting point of the drug Phensuximide is?
a) 72°C
b) 172°C
c) 272°C
d) 372°C
Q.3 Match the following with correct classifications of the drugs.
| i. Phensuximide | A. Inhalational anesthetics |
| ii. Oxazepam | B. ß-blockers |
| iii. Halothane | C. Sedative hypnotic |
| iv. Esmolol | D. Anticonvulsant drug |
a) i-A, ii-C, iii-D, iv-B
b) i-C, ii-A, iii-B, iv-D
c) i-D, ii-C, iii-A, iv-B
d) i-A, ii-D, iii-C, iv-B
Q.4 Mechanism of action of drug phensuximide includes?
I. Inhibition of neuronal systems that is important for generation of three per second rhythm.
II. Inhibition of depolarization-induced accumulation of cyclic adenosine monophosphate and GMP in brain tissues.
III. Inhibits releases of Acetylcholine from the presynaptic nerves.
IV. Antagonizes the muscarinic receptors.
a) I, IV
b) II, III
c) I, III, IV
d) I, II
Q.5 Correct sequence for True and False for the given statements related with the SAR of succinimide drugs?
- Phenyl substitution makes them active against electrically induced convulsion.
- N-methylation increases activity against electroshock seizures.
- N-methylation also increases the activity against chemically induced convulsions.
a) FFT
b) TFT
c) TFF
d) FFF
Q.6 Which drug is formed on reaction of phenylsuccinic acid with methylamine?
a) Halothane
b) Oxazepam
c) Phensuximide
d) Piperacetazine
Q.7 The drug Phensuximide is mainly used for?
a) Treatment of epilepsy
b) Treatment of pain due to gout
c) As an antiviral drug
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-a
3-c
4-d
5-b
6-c
7-a
REFERENCES
[1] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.