ISOFLURANE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Isoflurane
IUPAC nomenclature
(RS)-2-Chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane
Classification
Isoflurane is an inhalational anesthetic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 184.49 g/mol |
| 2 | Physical appearance | Clear colourless liquid |
| 3 | Boiling point | 48.5°C |
| 4 | Solubility | Solubility in water is poor |
| 5 | Octanol/water partition coefficient | 2.06 |
| 6 | Presence of ring | Not present |
| 7 | Number of chiral centers | 1 |
Mechanism of Action
- Isoflurane decreases the gap junction channel opening times and increases gap junction channel closing time which induces reduction in junctional conductance.
- The drug also increases the fluidity of the lipid membrane and thus, activates calcium dependent ATPase in the sarcoplasmic reticulum.
- Isoflurane also binds with D subunit of ATP synthase, GABA receptors, glutamate receptors , glycine receptors, the large conductance Calcium ion activated potassium channel and NADH dehydrogenase.
Structure Activity Relationship
The SAR for inhaled anesthetics can be summarized as follows:
- Halogenations of the hydrocarbons and ether increase the anesthetic potency.
- Halogenations of the hydrocarbons tends to induce arrhythmia in the following order F<Cl<Br<I.
- Due to presence of asymmetric halogenated carbon in ether, they are better anesthetics.
- Halogenated methyl ethyl ethers are more stable, more potent and have better clinical profile than halogenated diethyl ether.
- Fluorination increases the stability and decreases the flammability.
- Compete halogenations of the alkane or ether decreases the anesthetic potency and increases the convulsant potency
- Presence of double bond increases chemical reactivity and toxicity.
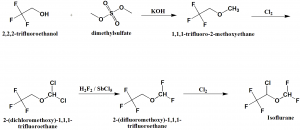
Method of synthesis
i. 2,2,2-trifluoroethanol undergoes methylation by dimethylsulfate to give methyl ether.
ii. Methyl ether is chlorinated by molecular chlorine to produce 2-(dichloromethoxy)-1,1,1-trifluoroethane.
iii. Chlorine atoms are replaced by fluorine atoms on subsequent interaction with hydrogen fluoride in presence of antimony(V) chloride.
iv. Resulting ether undergoes chlorination by molecular chlorine to produce isoflurane.
Therapeutic Uses
Isoflurane is used for:
- Induction and maintenance of general anesthesia in adult and pediatric patients.
Side Effects
Side effects of Isoflurane are:
- Cardiac disorders
- Hepatobiliary disorders
MCQs
Q.1 Choose the correct option related with the mechanism of action of drug Isoflurane?
a) Increases the gap junction channel opening time
b) Decreases the gap junction channel closing time
c) Increases the fluidity of the lipid membrane
d) All of the above
Q.2 Therapeutic use of drug Isoflurane is/are?
a) Control of seizures
b) As an anesthetic
c) Prevention of Diarrhea
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug Isoflurane?
I. Due to presence of asymmetric halogenated carbon in ether, they are lesser anesthetics.
II. Halogenated methyl ethyl ethers are less stable, less potent and have lesser clinical profile than halogenated diethyl ether.
III. Fluorination increases the stability and decreases the flammability.
IV. Compete halogenations of the alkane or ether decreases the anesthetic potency and increases the convulsant potency
a) I, IV
b) II, III
c) III, IV
d) I, III
Q.4 Number of chiral carbons present in the structure of isoflurane is?
a) 0
b) 1
c) 2
d) 3
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Isoflurane is?
I. Molecular weight = 184.49 gm/mol
II. Melting point = 140.2oC
III. White powder
IV. Ring structure absent
a) FFTF
b) TFFT
c) FTTF
d) TFFF
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Isoflurane: (RS)-2-Chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane.
II. Carvedilol: 1-(Aminomethyl)cyclohexaneacetic acid
III. Procyclidine: 8-chloro-6-(4-methylpiperazin-1-yl)benzo[b][1,4]benzoxazepine
IV. Codeine: 5-ethyl-5-pentan-2-yl-2-sulfanylidene-1,3-diazinane-4,6-dione
a) I
b) II, III, IV
c) II , III
d) III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Isoflurane | A. Carbamate derivative anticonvulsant drug |
| ii. 6-MP | B. Vinca alkaloids |
| iii. Vincristine | C. Ethylenimine |
| iv. Thiotepa | D. Purine antagonist |
a) i-B, ii-D, iii-C, iv-A
b) i-B, ii-C, iii-A, iv-D
c) i-C, ii-B, iii-D, iv-A
d) i-A, ii-D, iii-B, iv-C
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-c
2-b
3-c
4-b
5-b
6-a
7-d