BROMPHENIRAMINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
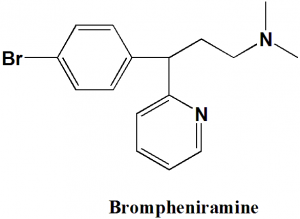
Brompheniramine
IUPAC nomenclature
(R/S)-3-(4-Bromophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine
Classification
- H1-receptor antihistamine
- Alkylamine antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 319.24 g/mol |
| 2 | Physical appearance | Yellow oily liquid |
| 3 | Melting point | <25oC |
| 4 | Solubility | Soluble in dilute acids |
| 5 | Octanol/water partition coefficient | 3.4 |
| 5 | Presence of ring | Phenyl, pyridine |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
i. Brompheniramine binds to histamine H1 receptors and blocks the action of endogenous histamine.
ii. It also has anticholinergic effects which is responsible for the side effects of drug, like drowsiness, sedation, dry mouth, etc.
Structure Activity Relationship
Structure activity of alkyl amines antihistamines can be summarized as:
- E- and Z- isomers in alkenes shows large difference in activity, where, E-isomers are more potent than Z-
- The two aromatic rings have different binding environments at the receptors.
- 5-6 angstrom distance is required between aromatic ring and tertiary aliphatic amine for biding at the receptor.
- S-enantiomers have greater affinity for H1 histamine receptors [1]
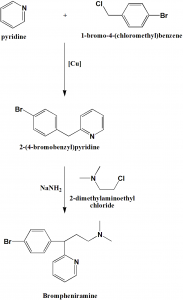
Method of synthesis
i. Pyridine undergoes alkylation by 4-bromobenzylcloride to give 2-(4-bromobenzyl)pyridine.
ii. Alkylation of the last with 2-dimethylaminoethylchloride in presence of sodium amide gives brompheniramine. [2]
Medicinal Uses
Brompheniramine is used for treatment of:
- Allergies
- Hay fever
- Common cold
- Watery eyes
- Runny nose
- Sneezing
- Cold symptoms
- Itchy
Side Effects
Side effects of Brompheniramine are:
- Seizures
- Loss of consciousness
- Hallucinations
- Drowsiness
- Dizziness
- Allergic reactions
- Confusion
- Nervousness
- Little urination
- Blurred vision
- Dry mouth/throat/nose
- Restlessness
MCQs
Q.1 Brompheniramine produces its effect through?
a) Binding with H1 receptors and producing agonizing effects
b) Binding with H1 receptors and producing antagonizing effects
c) Binding with H2 receptors and producing agonizing effects
d) Binding with H2 receptors and producing antagonizing effects
Q.2 Therapeutic use of drug Brompheniramine is/are?
a) Treatment of allergic symptoms
b) Treatment of runny nose
c) Treatment of sneezing
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of alkylamine antihistamine drugs?
I. E- and Z- isomers in alkenes shows large difference in activity, where, E-isomers are more potent than Z-isomers.
II. The two aromatic rings have different binding environments at the receptors.
III. 5-6 angstorm distance is required between aromatic ring and tertiary aliphatic amine for biding at the receptor.
IV. S-enantiomers have greater affinity for H1 histamine receptors
a) I , II, III, IV
b) II, IV
c) II, III
d) I, IV
Q.4 The starting chemicals required for the synthesis of drug brompheniramine are?
a) Pyridine and 4-bromobenzylchloride
b) Pyridine and phenyl magnesiumchloride
c) Pyrimidine and Ethanol
d) Purine and Antheranol
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug brompheniramine can be?
I. Molecular weight: 319.24 gm/mol
II. Melting point: <25oC
III. Octanol/water partition coefficient: 3.4
a) FTF
b) FTT
c) FFT
d) TTT
Q.6 Correct IUPAC nomenclature of drug Brompheniramine can be?
a) (R/S)-3-(4-Bromophenyl)-N,N-dimethyl-3-pyridin-2-yl-propan-1-amine
b) (R/S)-3-(4-Bromophenyl)-N,N-dimethyl-3-pyridin-2-yl-butan -1-amine
c) 2-[(4-Bromophenyl)-phenylmethoxy]-N,N-dimethylethanamine
d) 2-Bromo-2-chloro-1,1,1-trifluoroethane
Q.7 Match the following drugs with the correct number of chiral carbons they have in their structure:
| i. Brompheniramine | A. 0 |
| ii. Neostigmine | B. 2 |
| iii. Ephedrine | C. 7 |
| iv. Finasteride | D. 1 |
a. i-A, ii-D, iii-B, iv-C
b. i-C, ii-B, iii-D, iv-A
c. i-C, ii-A, iii-D, iv-B
d. i-D, ii-A, iii-B, iv-C
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-d
3-a
4-a
5-d
6-a
7-d
REFERENCES
[1] Lemke TL, Williams DA, editors. Foye’s principles of medicinal chemistry. Lippincott Williams & Wilkins; 2012 Jan 24. [2] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.