CYPROHEPTIDINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
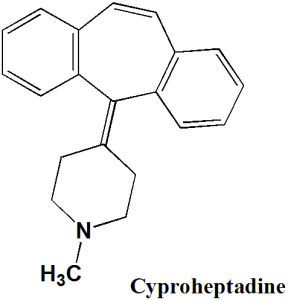
Cyproheptadine
IUPAC nomenclature
4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine
Classification
- H1-receptor antihistamine
- Piperidine derivative antihistamine drug
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 287.4 g/mol |
| 2 | Physical appearance | Crystals from dilute ethanol |
| 3 | Melting point | 112.8oC |
| 4 | Solubility | Soluble |
| 5 | Octanol/water partition coefficient | 4.69 |
| 5 | Presence of ring | Piperidine ring |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Cyproheptadine antagonizes the effect of histamine HA-receptors by competing with free histamine for binding at HA-receptor sites. This helps in reducing the negative symptoms occurring due to HA-receptor binding.
- The drug also competes with serotonin at receptor sites in smooth muscles of intestine and at other locations.
- Cyproheptadine also stimulates the appetite by antagonistic effects of serotonin on the appetite center of the hypothalamus.
Structure Activity Relationship
General structure activity of first generation H1-receptors antagonist can be summarized as:
- Ethylene chain gives maximum activity.
- Increasing or decreasing the chain length decreases the activity of drug, but promethazine is an exception.
- Chain may be present in saturated or unsaturated form, or sometimes a part of a ring system.
- Diaryl substitution is essential for significant H1 receptor affinity.
- Terminal nitrogen atom should be tertiary in nature.
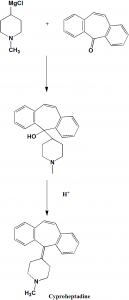
Method of synthesis
i. Reaction of 1-methyl-4-magnesiumchloropiperidine with 5H-dibeno[a,d]cyclohepten-5-one to form a carbinol.
ii. The formed carbinol is dehydrated under acidic condition to give cyproheptadine. [1]
Medicinal Uses
Cyproheptadine is used for treatment of:
- Allergic symptoms
- Sneezing
- Itching
- Bronchial asthma attacks
Side Effects
Side effects of cyproheptadine are:
- Dizziness
- Drowsiness
- Constipation
- Blurred vision
- Dry mouth
- Trouble swallowing
- Seizures
- Allergic reactions
MCQs
Q.1 Match the following with correct SAR of the first generation H1 receptor antihistamine drugs:
| i. Terminal nitrogen atom should be | A. Secondary in nature |
| ii. Ethylene chain gives | B. Tertiary in nature |
| C. Minimum activity | |
| D. Maximum activity |
a) i-A, ii-C
b) i-A, ii-D
c) i-B, ii-C
d) i-B, ii-D
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Cyproheptadine: 4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine
- Lomustine: 2-(diphenylmethoxy)-N,N-dimethylethanamine
- Lorcainide: (2R)-2-{2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine
- Felodipine: 4-hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-1λ6,2-benzothiazine-3-carboxamide
a) TFFT
b)TFFF
c) TTTT
d) FFFT
Q.3 Molecular weight of Cyproheptadine is?
a) 245.3 gm/mol
b) 452.0 gm/mol
c) 287.4 gm/mol
d) 46.9 gm/mol
Q.4 Along with antihistaminic property, Cyproheptadine also has?
a) Anti-metabolic activity
b) Anti-neoplastic actvity
c) Anti-serotonergic activity
d) Anti-hyperlipidemic activity
Q.5 Which amongst the following is not a therapeutic use of drug Cyproheptadine?
a) Chronic urticaria
b) Bronchial asthma attacks
c) Itching
d) Anesthetics
Q.6 Which of the following drug and their classification are correct?
I. Cyproheptadine: Proton pump inhibitor
II. Cocaine: Local anesthetics
III. Rabeprazole: Proton pump inhibitor
IV. Amyl nitrite: Vasodilator
a) I, III
b) II, III, IV
c) III, IV
d) I, II, III
Q.7 Number of chiral carbons present in the structure of Cyproheptadine?
a) 1
b) 3
c) 4
d) 0
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-d
2-b
3-c
4-c
5-d
6-b
7-d
REFERENCES
[1] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.