TRIMEPRAZINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
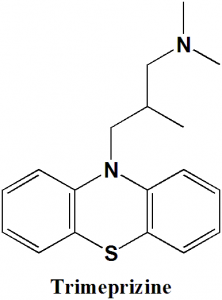
Trimeprazine
Or
Alimemazine
IUPAC nomenclature
10-(3-dimethylamino-2-methylpropylphenothiazine
Classification
- H1-receptor antihistamine
- Phenothiazine derivative antihistamine drug
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 298.4 g/mol |
| 2 | Physical appearance | Crystals |
| 3 | Melting point | 68oC |
| 4 | Solubility | 0.942 mg/ml |
| 5 | Octanol/water partition coefficient | 4.6 |
| 5 | Presence of ring | Phenothiazine |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
- Trimeprazine antagonizes the effect of histamine HA-receptors by competing with free histamine for binding at HA-receptor sites. This helps in reducing the negative symtoms occurring due to HA-receptor binding.
Structure Activity Relationship
Structure activity of phenothiazine derivatives antihistamines can be summarized as:
- There are 2-3branched alkyl chain between the ring system and the nitrogen atom.
- On unbranching, antipsychotic series of drugs can be obtained.
General structure activity of first generation H1-receptors antagonist can be summarized as:
- Ethylene chain gives maximum activity.
- Increasing or decreasing the chain length decreases the activity of drug, but promethazine is an exception.
- Chain may bepresent in saturated or unsaturated form, or sometimes a part of a ring system.
- Diaryl substitution is essential for significant H1 receptor affinity.
- Terminal nitrogen atom should be tertiary in nature.
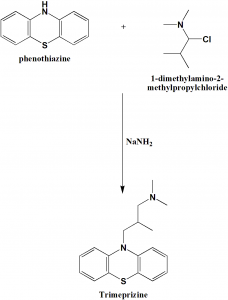
Method of synthesis
Alkylation of phenothiazine with 1-dimethylamino-2-methyl-propylchloride gives trimeprazine. [1]
Medicinal Uses
Trimeprazine is used for treatment of:
- Itching
- Relieve in coughing
Side Effects
Side effects of Trimeprazine are:
- Dizziness
- Upset stomach
- Abdominal pain
- Trouble swallowing
- Seizures
- Dizziness
- Increased heartbeat
- Allergic reactions
MCQs
Q.1 What can be the correct IUPAC nomenclature of Trimeprazine?
a) 2-(diphenylmethoxy)-N,N-dimethylethanamine
b) 10-(3-dimethylamino-2-methylpropylphenothiazine
c) (RS)-1-[(4-chlorophenyl)- phenyl-methyl]-4- [(4-tert-butylphenyl) methyl] piperazine
d) (R)-(+)-2-([3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methylsulfinyl)-imidazole
Q.2 Which amongst the following statements is/are incorrect related to the Phenothiazine derivative antihistamine drugs?
I. There are 2-3branched alkyl chain between the ring system and the nitrogen atom.
II. On unbranching, antipsychotic series of drugs can be obtained.
a) I
b) I, II
c) II
d) None
Q.3 Types of rings present in the structure of Trimeprazine?
I. Imidazole
II. Pyridine
III. Phenothiazine
IV. Phenyl
a) I, II
b) I, IV
c) II, IV
d) III
Q.4 Side effects of drug Trimeprazine is/are?
a) Allergic reactions
b) Trouble swallowing
c) Upset stomach
d) All of the above
Q.5 Match the following drugs with their correct molecular weight-
| i. Trimeprazine | A. 166.24 gm/mol |
| ii. Pyrrobutamine | B.240.34 gm/mol |
| iii. Edrophonium | C. 298.4 gm/mol |
| iv. Pheniramine | D. 311.8 gm/mol |
a) i-A, ii-B, iii-C, iv-D
b) i-C, ii-A, iii-B, iv-D
c) i-C, ii-D, iii-A, iv-B
d) i-A, ii-C, iii-D, iv-B
Q.6 An example of drug from class phenothiazine derivative antihistamine drug?
a) Trimeprizine
b) Besoprolol
c) Hydroxyamphetamine
d) Ephedrin
Q.7 Octanol/water partition coefficient of Trimeprazine is?
a) 7.3
b) 5.3
c) 4.6
d) 1.6
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-b
2-d
3-d
4-d
5-c
6-a
7-c
REFERENCES
[1] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.