CETIRIZINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
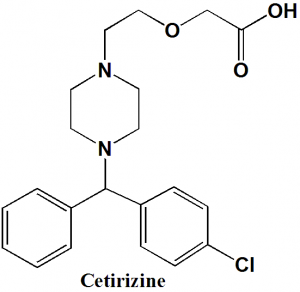
Cetirizine
IUPAC nomenclature
(±)-[2-[4-[(4-Chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic acid.
Classification
- H1-receptor antihistamine
- Piperazine antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 389.9 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 110-115oC |
| 4 | Solubility | 101 mg/L |
| 5 | Octanol/water partition coefficient | 2.8 |
| 5 | Presence of ring | Piperazine, phenyl |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
- Cetirizine is a metabolite of hydroxyzine.
- It selectively inhibits peripheral H1 receptors.
Structure Activity Relationship
Structure activity of piperizine antihistamines can be summarized as:
- These are the derivatives of ethylene diamines.
- The connecting moiety is CHN group
- Primary structural difference is nature of para aromatic ring substituent
- These are moderately potent.
- Slow onset of action
- Low incidence of drowsiness
- They also exhibit peripheral and central antimuscarinic activity.
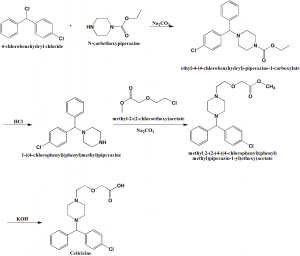
Method of synthesis
i. 4-chlorobenzhydryl chloride is reacted with N-carbethoxypiperazine in presence of sodium carobonate to give ethyl-4-(4-chlorobenzhydryl)-piperazine-1-carboxylate.
ii. The above formed compound is treated with hydrochloric acid to get 1-(4-chlorodiphenylmethyl)piperazine.
iii. The last is reacted with methyl 2-(2-chloroethoxy)acetate in presence of sodium carbonate to give methyl 2-[2-[4[(4-chlorodiphenylmethyl)-1-piperazinyl]ethoxy]acetate.
iv. On treating the above formed compound with KOH produces cetirizine. [1]
Medicinal Uses
Cetirizine is used for treatment of:
- Allergy symptoms
- Watery eyes
- Runny nose
- Itching
- Sneezing
- Hives
Side Effects
Side effects of Cetirizine are:
- Drowsiness
- Tiredness
- Dry mouth
- Stomach pain
MCQs
Q.1 Mechanism of action of Cetirizine drug includes?
a) Binds with H2 receptor
b) Conversion into hydroxyzine
c) Vasoconstriction
d) Agonist effects H1 receptor for Histamine
Q.2 Medicinal use of drug Cetirizine is/are?
a) Allergy symptoms
b) Watery eyes
c) Sneezing
d) All of the above
Q.3 Select the correct option from below related with the SAR of piperazine antihistamines-
a) These are derivatives of Pyrimidines
b) They have low incidence of drowsiness
c) They are highly potent
d) They exhibit very fast onset of action
Q.4 Number of chiral carbons present in the structure of Cetirizine is?
a) 0
b) 1
c) 2
d) 3
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Cetirizine can be?
- Molecular weight: 652.3 gm/mol
- Physical appearance: Oil
- Octanol/water partition coefficient: 2.8
a) TTT
b) TFT
c) FFT
d) TFF
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Lansoprazole: 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine
II. Hydroxyaphetamine: (RS)-2-([4-(3-Methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl)-1H-benzo[d]imidazole.
III. Cetirizine: (±)-[2-[4-[(4-Chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic acid
IV. Triprolidine: 2-[(E)-1-(4-methylphenyl)-3-pyrrolidin-1-yl-prop-1-enyl]pyridine
a) I, II
b) III, IV
c) IV
d) I, II, III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Cetirizine | A. H1-receptor antihistamine |
| ii. Acetaminophen | B. Sedative-hypnotic |
| iii. Nalorphine | C. Anti-inflammatory |
| iv. Alprazolam | D. Narcotic antagonist |
a) i-A, ii-C, iii-D, iv-B
b) i-D, ii-A, iii-C, iv-B
c) i-D, ii-B, iii-A, iv-C
d) i-B, ii-D, iii-A, iv-C
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-b
2-d
3-b
4-b
5-c
6-b
7-a
REFERENCES
[1] EP 58 146 (UCB; appl. 5.2.1982; GB-prior. 6.2.1981, 8.4.1981).