Acid-Base balance In Urinary System and MCQ for NEET, GPAT CSIR NET JRF and GATE Lifesciences Exam
Acid-base balance is all about maintaining the H+ ion concentration(pH) at an appropriate level. This is important for normal cellular function. The pH of blood is 7.35-7.45. To maintain this range, three major mechanisms takes place in the body-
1. BUFFER SYSTEMS
Buffer acts to bind H+ ions, thus removes the highly reactive excess hydrogen ions from the solution. Buffers thus raise the pH, but do not remove H+ from the body. There are 3 buffer systems-
Protein buffer system
This type of buffer is found mostly in the intracellular fluid and blood plasma. The hemoglobin is a buffer within RBCs and albumins is a main protein buffer in blood plasma.
When the pH rises, following changes occur in the structure of protein. The released H+ ion reacts with the OH- ion(since pH rises) and forms water

When the pH falls and become acidic, following changes occur in its structure. The free amino group at the other end of a protein acts as a base reacting with H+ ion.
Since hemoglobin acts as a buffer for RBCs, so when blood flows through the systemic capillaries, The CO2 present in the tissue cells enters the RBCs and reacts with water to form carbonic acid(H2CO3). after the formation of H2CO3, it dissociates to form H+ and HCO3-. In the tissue cells when CO2 enters the RBCs at the same tine oxy-hemoglobin bond also breaks and hemoglobin picks up most of the H+ ion and forms Hb-H.
Carbonic Acid-Bicarbonate buffer system
It is based on two structures, carbonate ion(HCO3-) and carbonic acid(H2CO3).
If the Ph rises, the following reaction takes place. The carbonic acid thus formed dissociates into CO2 and water; The CO2 formed is exhaled through lungs
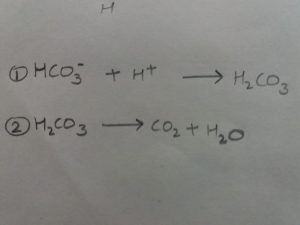
If the pH falls, the carbonic acid dissociates and following reaction occur.
Phosphate Buffer system
It is mainly important in cytosol of the cell and in urine. It is based on two structure: dihydrogen phosphate(H2PO4-) and mono-hydrogen phosphate.
If the Ph rises, the OH- ion reacts with dihydrogen phosphate; and when Ph falls, H+ ion combines with mon-hydrogen phosphate. the reactions are as follows.
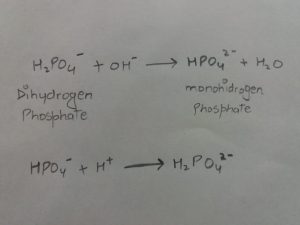
2.EXHALATION OF CO2
When the pH of blood rises, then the chemoreceptors present in the medulla oblongata, aortic sinus and carotid sinus are stimulated. as these chemoreceptors are stimulated, they send nerve impulses to the inspiratory area of medulla oblongata which results in contraction of diaphragm. These contractions further facilitates exhalations of CO2, and less carbonic acid is formed. Thus Ph of blood comes back to normal.
3.KIDNEY EXCRETION OF H+
The proximal convoluted tubules(PCT) present in the nephrons contains Na+/H+ antiporters. these antiporters reabsorbs Na ions and secretes H+. Thus Ph of urine becomes acidic due to increased concentration of H+ ions.
Also the intercalated cells of the collecting duct of nephrons reabsorbs the potassium ion and carbonate ions and secrete H+ ions and Ph becomes acidic. Some intercalated cells also secretes the carbonate ions because of which the Ph of urine becomes basic. In this way, the Ph of urine is maintained in the body.
Multiple choice questions(MCQs)
1. What is the normal Ph range of blood?
A. 8.3-9.5 b. 7.3-7.
C. 5.5-5.8 D. 3.4-3.5
2. Which mechanism is not the part of acid base balance?
A. exhalation of CO2 B. inhalation of CO2
C. buffer system D. kidney mechanism
3. Which structures forms the basis of phosphate buffer system?
A. dihydrogen phosphate B. carbonic acid
C. mono-hydrogen phosphate D. both A and C
4. Which of the following buffers functions for buffer systems for maintaining Ph?
A. protein buffer B. carbonic-bicarbonate buffer
C. phosphate buffer D. all of the above
5. Which of the following statement is NOT true?
A. intercalated cells do not secrete carbonate ions
B. dissociation of carbonic acid forms CO2
C. OH ions reacts with dihydrogen phosphate
D. Ph becomes basic when carbonate ions are secreted
6. What acts as a buffer within RBCs?
A. hemoglobin B. water
C. oxygen D. CO2
7. Match the following-
a. protein buffer 1. chemoreceptors
b. phosphate buffer 2. Sodium-hydrogen antiporters
C. PCT 3. Cytosol and urine
D. exhalation of CO2 4. Intracellular fluid and blood
8. In which region of brain, the inspiratory area lies?
A. pons B. third ventricle
C. medulla oblongata D. fourth ventricle
9. Where are the Na/H+ antiporters present?
A. PCT B. DCT
C. collecting duct D. loop of henle
10. In which regions, the chemoreceptors for exhalation of CO2 mechanism NOT present?
A. medulla oblongata B. aortic sinus
C. carotid sinus D. none of the above
ANSWERS:-
1. 7.3-7.4
2. inhalation of CO2
3. both A and C
4. all of the above
5. intercalated cells do not secrete carbonate ion
6. hemoglobin
7. a – 4 b – 3 c – 2 d – 1
8. medulla oblongata
9. PCT
10. none of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
REFERENCE:-Gerard J. Tortora -Principles of anatomy and physiology; edition twelfth ; page no.-: 1070-1075.