ACID-BASE Titration PART-2: Types, End Point Detection and MCQ for GPAT, GATE and CSIR NET JRF
Based on the nature of acid and base neutralization reactions are classified as :-
1.Strong acid & strong base
HCL +NaoH → NaCl + H2O
2.Strong acid & Weak base
HCl + NH4OH → NH4Cl +H2o
3. Weak acid & Strong base
CH3COOH + NaOH → CH3COONa +H2O
4. Weak acid & Weak base
CH3COOH + NH4OH → CH3COONH4 +H2O
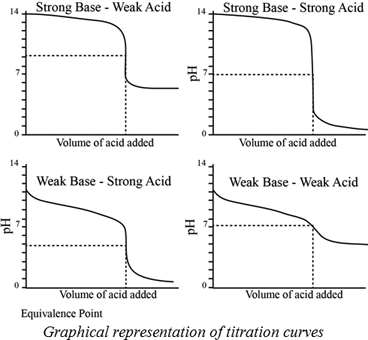
TITRATION :-
There are mainly two types of titration are in acid base titrations
- ACIDIMETRY
- ALKALIMETRY
1. ACIDIMETRY :-
Estimating an alkali solutions with a standard acid solutions is known as acidimetry. It is classified in two types
-
Direct titration :- Acid is titrated by base. (Acid in burette base in conical flask)
-
Residual titration :- Base reacts with large conc. of acid.Unreacted amount of acid is titrated with base.
2. ALKALIMETRY :-
Estimating an acidic solutions with a standard base solutions is known as alkalimetry. It is classified in two types
-
Direct titration :- Base is titrated with acid. (Base in burette acid in conical flask)
-
Residual titration :- acid reacts with large conc. of base.Unreacted amount of base is titrated with acid.
Direct titration (Acidimetry) :-
eg. titration of Na2CO3 with HCl.
2HCl + Na2CO3 → 2NaCl + CO2 +H2O
Direct titration (Alkalimetry) :-
e.g. Assay of boric acid
H3BO3 +NaOH → NaBO2 + 2H2O
END-POINT DETECTION :-
1. Neutralization indicator :-
A. Ostwald theory :– The undissosiated indicator acid or a base has a color different than its own.
| Titration Between | pH at end point | Commonly used indicator |
| Weak acid and strong base | Alkaline range | Thymol Blue, Phenolphthalein |
| Weak base and strong acid | Acidic range | Methyl orange, Methyl red |
B. Resonance Theory :- The difference in color of the same compound in acid and base medium is apparently due to difference in structure of two forms.
| pH range | Acid | Base | |
| Thymol blue | 1.2-2.8 | red | yellow |
| Bromocresol blue | 3.0-4.6 | yellow | blue |
| Methyl orange | 3.1-4.4 | red | orange |
| Methyl red | 4.2-6.6 | red | yellow |
| Phenol red | 6.8-8.4 | yellow | Red |
| Phenolphthalein | 8.3-11.0 | colorless | Red |
2. mixed indicator
3.universal indicator
Buffer solutions :- A solutions of substance or a mixture of substance which help in maintaing and establishing a specific pH.
pH = pka + log [conjugated base]/[conjugated acid]
This is Henderson-Hasselbalch equation which relates pH of solutions containing comparable and appreciable conc. of a conjugate acid-base pair to ratio of their concentrations.
MCQ
1. Assay of boric acid is done by
a.Direct titration (Alkalimetry)
b.Direct titration (Acidimetry)
c. a and b
d. None of the above
2. Resonance Theory is
a.The same in color of the same compound in acid and base medium is apparently due to difference in structure of two forms.
b.The difference in color of the same compound in acid and base medium is apparently due to same in structure of two forms.
c.The difference in color of the different compound in acid and base medium is apparently due to difference in structure of two forms.
d.The difference in color of the same compound in acid and base medium is apparently due to difference in structure of two forms.
3.In acid base titration at pH 6.8-8.4 which indicator are used ?
a.Bromocresol blue
b.Phenol red
c.Methyl orange
d.Methyl red
4. Methyl red is give red colour at which pH ?
a.1.2-3.5
b.4.2-6.6
c.7.8-8.9
d.9.8-10.2
5. When titration occur between Weak acid and strong base which indicator are used ?
a.Thymol Blue
b. Phenolphthalein
c. a and b
d.None of the above
6.Which sentence is false about Henderson-Hasselbalch equation ?
a. It is used to determine pKa value of solutions.
b. pH of solutions containing comparable and appreciable conc. of a conjugate acid-base pair to ratio of their concentrations.
c. It is used to calculate valency of molecule.
d.pH = pka + log [conjugated base]/[conjugated acid]
7.When titration occur between Weak base and strong acid which indicator are used ?
a.Methyl orange
b.Methyl red
c. a and b
d. None of the above
8. Which sentence is false about indicator ?
a.Phenolphthalein give colourless solutions at pH 8.3-11.0 in acidic medium.
b.Methyl red give red solutions at pH 4.2-6.6 in acidic medium.
c.Methyl orange give orange solutions at pH 3.1-4.4 in base medium.
d.Methyl red give red solutions at pH 4.2-6.6 in basic medium.
ANSWER KEY
1.A
2.D
3.B
4.B
5.C
6.C
7.C
8.D
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in Online FREE GATE TEST: CLICK HERE
Participate in Online FREE CSIR JET JRF TEST: CLICK HERE
REFERENCE :-
VOGEL′S TEXT BOOK OF QUANTITATIVE CHEMICAL ANALYSIS FIFTH EDITION BY G.H.JEFERY, J.BASSETT (PG.NO. 262-278)