ALPRAZOLAM Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
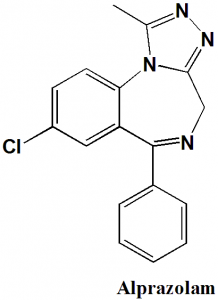
Alprazolam
IUPAC nomenclature
8-Chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a] [1,4]benzodiazepine.
Classification
Alprazolam is a benzodiazepine sedative-hypnotic.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 308.8 g/mol |
| 2 | Physical appearance | Solid; crystals from ethyl acetate |
| 3 | Melting point | 228-229°C |
| 4 | Octanol/water partition coefficient | 2.12 |
| 5 | Solubility | 13.1 mg/ml in water |
| 6 | Presence of ring | Diazepine, benzene, triazole |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
i. Alprazolam acts on benzodiazepine receptors BNZ1 and BNZ2.
ii. Alprazolam converts into two active metabolites: 4-hydroxylprazolam and α-hydroxyprazolam. The former metabolite acts with 0.20 times the potency of alprazolam, while the latter metabolite acts with 0.66 times the potency of the alprazolam.
iii. Action on BNZ1 leads to sedation and anti-anxiety effects, while action on BNZ2 produces effects on memory, coordination, muscle relaxation and anticonvulsive activities.
iv. A calming effect is also produced by the drug, when it binds with GABAA receptors, which increases the GABA binding to the receptors, which leads to inhibition of nervous system.
Structure Activity Relationship
- Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
- An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
- Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
- When Heterocycles used as ring A, drug shows poor pharmacological activity.
- A proton-accepting group is essential on Ring B for binding with GABAA
- When the proton accepting group is present on the 2-position of the ring B, and is in coplanar spatial orientation with Ring A, maximum activity is observed.
- Replacement of oxygen with sulfur in ring B results in alteration in the selectivity for binding with GABA BZR subpopulations, but anxiolytic properties are maintained.
- There is no effect on agonist activity, but the antagonist activity dereases when methylene 3-position or imine nitrogen of the ring B is substituted.
- Derivatives having the 3-hydroxy moiety are fast excreted.
- Sterically large substituents on ring B , like tert-butyl group reduces the receptor affinity and the in vivo activity.
- 4,5-double bond and 4-position nitrogen is not essential for anxiolystic activity.
- BZR affinity is decreased if C=N bond is replaced with C-N bond.
- 5-phenyl ring C is not necessary for the binding with BZR.
- Substitution at the para position of the ring C decreases the agonist activity of the drug.
- There is no change observed in the agonist property of the drug when there is substitution at ortho position.
- When 1,2-bond f the ring C is annelated with an additional electron rich ring such as imidazole, affinity of the BZR increases. [1]
Method of synthesis
i. Reaction of 2,6,-dichloro-4-phenylquinoline (1) with hydrazine (2) gives 6-chloro-2-hydrazino-4-phenylquinoline (3).
ii. On boiling (3) with triethyl orthoacetate (4) in xylene eads to heterocyclization to form a triazole derivative (5).
iii. (5) undergoes oxidative cleavage by using sodium periodate (6) and Ruthenium dioxide (7) in an acetone-water system to form 2-[4-(3′-methyl-1,2,4-triazolo)]-5-chlorobenzophenone (8).
iv. On oxymethylation of the (8) using formaldehyde (9), followed by substitution of hydroxyl group by phosphorous tribromide (10) gives 2-[4-(3′-methyl-5′-bromomethyl-1,2,4-triazolo)]-5-chlorobenzophenone (11).
v. On substitution of the bromine atom with an amino group using ammonia and spontaneous heterocyclization produces alprazolam. [2]
Therapeutic Uses
Alprazolam is used for:
- Treatment of anxiety
- Treatment of panic disorders
Side Effects
Side effects of alprazolam are:
- Dizziness
- Drowsiness
- Change in libido
- Increased salivation
- Lightheadedness
- Mood changes
- Hallucinations
- Suicidal thoughts
- Loss of coordination
- Speech problems
- Memory problems
- Pale skin
- Pale eyes
MCQ
Q.1 Binding of alprazolam with acetylcholine muscarinic receptors results in?
a) Agonizing effect on muscarinic receptors
b) Antagonizing effect on muscarinic receptors
c) Do not produce any significant effect
d) Do not bind with muscarinic receptor
Q.2 Therapeutic use of drug Alprazolam is/are?
a) Treatment of Acute alcohol withdrawal
b) Treatment of seizures
c) Treatment of anxiety
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug Alprazolam?
I. Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
II. An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
III. Substitutions at 6, 8 or 9 position with electronegative group on ring A will decrease the functional anxiolytic activity.
IV. When Heterocycles used as ring A, drug shows poor pharmacological activity.
a) I, IV
b) I, II, IV
c) I, II, III, IV
d) II, III, IV
Q.4 Type of ring structures present in the structure of Alprazolam?
a) Triazole
b) Benzene
c) Diazepine
d) All of the above
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug solifenacin?
I. Molecular weight is 308.8 gm/mol
II. Produces crystals from ethylacetate
III. Melting point is between 228-229°C
IV. Diazepine ring is present
a) TFTF
b) TTTT
c) FFFF
d)FFFT
Q.6 Correct statements for the IUPAC nomenclatures of the are?
I. Solifenacin: (3R)-1-Azabicyclo[2 2 2]oct-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate
II. Zaleplon: N-(3-(3-cyanopyrazolo[1,5-a] pyrimidin-7-yl)phenyl)-N-ethylacetamide
III. Alprazolam: 7-Chloro-1,3-dihydro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one
IV. Diazepam: 8-Chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a] [1,4]benzodiazepine
a) II, IV
b) I, II
c) I, III, IV
d) I, II, III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Solifenacin | A. Barbiturate sedative-hypnotic |
| ii. Thiobarbital | B. Benzodiazepine sedative-hypnotic |
| iii. Alprazolam | C. Acetylcholine antagonist |
| iv. Zaleplon | D. Nonbenzodiazepine sedative-hypnotic |
a) i-C, ii-A, iii-B, iv-D
b) i-B, ii-C, iii-A, iv-D
c) i-D, ii-A, iii-C, iv-B
d) i-A, ii-C, iii-B, iv-D
Participate in Free Online Test for GPAT, Pharmacist,Drug Inspector
ANSWERS
1-d
2-c
3-c
4-d
5-b
6-b
7-a