ZOLPIDEM Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
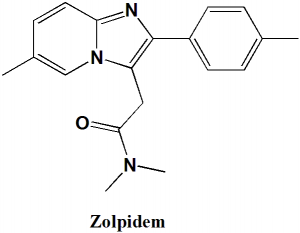
Zolpidem
IUPAC nomenclature
N,N-Dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide hemitartrate
Classification
Zolpidem is a nonbenzodiazepine GABAA agonist
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 307.4 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 196°C |
| 4 | Octanol/water partition coefficient | 3.02 |
| 5 | Solubility | 23 mg/ml |
| 6 | Presence of ring | Imidazopyridine, benzene |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Zolpidem interacts with a GABA-NZ receptor complex.
- The sedative, anticonvulsive, anxiolytic and myorelaxing properties of the drug is due to binding with GABAA receptor chloride channel macromolecular complex.
- Zolpidem specifically binds with α1/α5 According to the recent studies, zolpidem primary binds with α-1,2, and 3 subunits of GABA receptor and not with the α5 subunit.
Structure Activity Relationship
- Conversion of either of the imidazole nitrogen into hydrogen bond donors leads to the loss of the selectivity for a1 subtype GABAA
- When imidazole of zolpidem is converted into azaisostere congener, there is no effect in binding to α1-subtype receptors, but there is decrease in binding with α2 and α3 receptors
- Antiplanar group is critical in facilitating binding to GABAA
- Antiplanar group provides binding selectivity and affinity. [1]
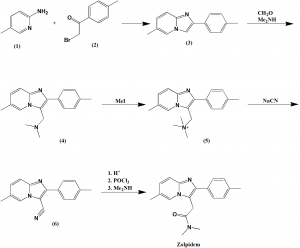
Method of synthesis
i. Reaction of 2-amino-5-methylpyridine (1) with 2-bromo-4-methylacetophenone (2) to give imidazopyridine (3).
ii. (3) undergoes aminomethylation to form amine (4)
iii. (4) is methylated with methyliodide and leads to formation of quaternary ammonium salt (5).
iv. Reaction of (5) with sodium cyanide produces nitrile (6).
v. (6) on acidinc hhydrolysis gets convert into corresponding acid chloride which on heating with dimethylamine produces zolpidem. [2]
Therapeutic Uses
Zolpidem is used for:
- Treatment of insomnia
Side Effects
Side effects of Zolpidem are:
- Dizziness
- Mood changes
- Hallucinations
- Suicidal thoughts
- Depression
- Aggressive behavior
MCQ
Q.1 What can be the correct IUPAC nomenclature of Zolpidem?
a) (S)-2-[1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl] pyrrolidin-3-yl] -2,2-diphenyl-acetamide
b) (S)-2-[3-(Diisopropylamino)-1-phenylpropyl]-4-methylphenol.
c) 7-Chloro-2,3-dihydro-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3-carboxylic acid
d) N,N-Dimethyl-2-[6-methyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetamide hemitartrate
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Zolpidem?
I. Ring A should include an aromatic or heteroaromatic ring for binding with 5-phenyl-1,4-benzodiazepin-2-one derivatives.
II. An electronegative group at 7-position of the ring A increases the functional anxiolytic activity.
III. Substitutions at 6, 8 or 9 position with electronegative group on ring A will increase the functional anxiolytic activity.
IV. When Heterocycles used as ring A, drug shows poor pharmacological activity.
a) I, II
b) III
c) III, IV
d) II
Q.3 Corrects sequence of the steps involved in the synthesis of Zolpidem from 2-amino-5-methylpyridine?
I. Aminomethylation
II. Methylation
III. Reaction with 2-bromo-4-methylacetophenone
IV. Acidic hydrolysis
V. Reaction with sodium cyanide
a) III- II – I- IV – V
b) II – I – III- IV – V
c) III – I – II – V – IV
d) I – III – II- V – IV
Q.4 Side effects of drug Zolpidem is/are?
a) Hallucinations
b) Dizziness
c) Depression
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Darifenacin | A.426.5 gm/mol |
| ii. Tolterodine | B.307.4gm/mol |
| iii. Chlorazepate | C. 325.5 gm/mol |
| iv. Zolpidem | D. 314.72 gm/mol |
a) i-C, ii-B, iii-A, iv-D
b) i-C, ii-B, iii-D, iv-A
c) i-A, ii-C, iii-D, iv-B
d) i-B, ii-A, iii-D, iv-C
Q.6 An example of drug from class nonbezodiazepine sedative hypnotic?
a) Methysergide
b) Zolpidem
c) Amphetamine
d) Tolterodine
Q.7 The type of ring system found in the structure of Chlorazepate?
a) Dihydrobenzofurane
b) Pyrrolopyrimidine
c) Imidazopyridine
d) Pyrrolopyrrole ring
Participate in Free Online Test for GPAT, Pharmacist,Drug Inspector
ANSWERS
1-d
2-b
3-c
4-d
5-c
6-b
7-c