AMLODIPINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Amlodipine
IUPAC nomenclature
3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate.
Classification
- Dihydropyridines
- Calcium channel blockers
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 408.9 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 199-201oC |
| 4 | Solubility | Slightly soluble in water |
| 5 | Octanol/water partition coefficient | 3 |
| 5 | Presence of ring | Dihydropyridine, phenyl |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
- Amlodipine blocks the entry of calcium ions into vascular smooth muscles and cardiac muscles at the time of depolarization.
- Amplodipine can bind with dihydropyridine and nondihydropyridine receptor sites present on the cell membrane.
- There is stronger effect of this drug on vascular smooth muscles than on cardiac muscles which helps in reducing the peripheral vascular resistance, which results in lowering the blood pressure.
Structure Activity Relationship
General structure activity of dihydropyridines calcium channel blockers can be summarized as:
- R1 should be unsubstituted.
- R1 should be easily detachable group.
- Basic amino ethyl ether chain at R2 increases the potency of drug, while H/aryl group results in loss of activity of drug.
- Substitution of R3 and R5 with alkoxy carbonyl group gives optimum activity.
- Branching of alkyl chain of ester group decreases the activity.
- R4 must be phenyl ring.
- S-enantiomers are more active than R-enantiomers.
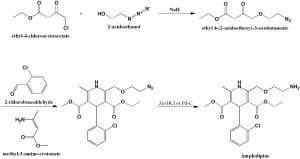
Method of synthesis
i. Ethyl-4-chloroacetoacetate reacts with 3-azidoethanol in presence of sodium hydride to give ethyl-4—(2-azidoethoxy)-acetoacetate.
ii. The last reacts with 2-chlorobenzaldehyde and methyl-3-aminocrotonate to give 3-ethyl-5-methyl-2-[(-azidoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinecarboxylate.
iii. On reduction of the above formed compound, amplodipine is formed. [1]
Medicinal Uses
Amlodipine is used for the treatment of:
- Coronary artery disease
- Chronic stable angina
- Vasospastic angina
- Hypertension
- Prevention of heart attacks
Side Effects
Side effects of Amlodipine are:
- Dizziness
- Weakness
- Flushing
- Fainting
- Lightheadedness
- Swelling of ankles
- Constipation
- Headache
- Allergic reactions
MCQs
Q.1 “3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate” is the IUPAC nomenclature of which drug?
a) Carvedilol
b) Pentazocine
c) Amlodipine
d) Tropicamide
Q.2 Melting point of drug Amlodipine is?
a) 408.9oC
b) 199-201 oC
c) 345 oC
d) 741 oC
Q.3 Match the following with correct classifications of the drugs.
| i. Amplodipine | A. Narcotic antagonist |
| ii. Phenacemide | B. Anticonvulsant |
| iii. Pentobarbital | C. Sedative-hypnotics |
| iv. Nalorphine | D. Calcium channel blocker |
a) i-C, ii-A, iii-D, iv-B
b) i-D, ii-C, iii-A, iv-B
c) i-D, ii-B, iii-C, iv-A
d) i-C, ii-D, iii-B, iv-A
Q.4 Mechanism of action of the drug Amlodipine includes?
I. Amlodipine blocks the entry of calcium ions into vascular smooth muscles and cardiac muscles at the time of depolarization.
II. Amplodipine can bind with dihydropyridine and nondihydropyridine receptor sites present on the cell membrane.
III. There is stronger effect of this drug on vascular smooth muscles than on cardiac muscles which helps in reducing the peripheral vascular esistance, which results in lowering the blood pressure.
a) I, II, III
b) II, III
c) I, III
d) I, II
Q.5 Correct sequence for True and False for the given statements related with the SAR of Thiazide diuretics can be?
- R1 should be unsubstituted.
- R1 should be easily detachable group.
- Basic amino ethyl ether chain at R2 increases the potency of drug, while H/aryl group results in loss of activity of drug
a) TFF
b) FFT
c) TTT
d) FFF
Q.6 Number of chiral carbons present in the structure of amlodipine is?
a) 0
b) 1
c) 2
d) 3
Q.7 The drug amlodipine is mainly used for?
a) Chronic stable angina
b) Treatment of hypertesnsion
c) Treatment of vasospastic angina
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-c
2-b
3-c
4-a
5-c
6-b
7-d
REFERENCES
[1] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.