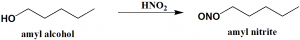AMYL NITRITE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
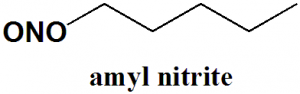
Amyl nitrite
IUPAC nomenclature
(3-methylbutyl) nitrite
Classification
- Organic nitrate antianginal drug
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 117.15 g/mol |
| 2 | Physical appearance | Clear colorless to yellowish liquid |
| 3 | Boiling point | 97-99oC |
| 4 | Solubility | Almost insoluble in water |
| 5 | Octanol/water partition coefficient | log Kow = 2.85 |
| 5 | Presence of ring | Not present |
| 6 | Number of chiral centers | Not present |
Mechanism of Action
- Amyl nitrite is the source of NO which reduces systemic pulmonary arterial pressure and decreases cardiac output through peripheral vasodilation
- Amyl nitrite can be used in cyanide poisoning as it combines with cyanide to form cyanamethemoglobin which is a nontoxic compound.
Structure Activity Relationship
General structure activity of organic nitrate antianginal drugs be summarized as:
- The number of nitrate groups determines the potency of organic nitrate for guanylate cyclase activation.
- Increase in nitric group increases the potency.
- Increase in lipophillicity doesn’t have major effect over activation of drug.
Method of synthesis
Amyl alcohol reacts with HNO2 to produce amyl nitrite. [1]
Medicinal Uses
Amyl nitrite is used for treatment and prevention of:
- Angina pectoris
Side Effects
Side effects of Amyl nitrite are:
- Dizziness
- Headache
- Flushing
- Syncope
- Hypotension
- Tachycardia
- Methemoglobinemia
- Nausea
- Vomiting
- Weakness
- Restlessness
- Uncontrolled urination
MCQs
Q.1 Amyl nitrite is the source of?
a) Sodium nitrate
b) Sodium nitride
c) Sodium nitrite
d) Sodium hydride
Q.2 Therapeutic use of drug amyl nitrite is/are?
a) Treatment of angina pectoris
b) Treatment of methemoglobinemia
c) Treatment of syncope
d) All of the above
Q.3 What is the effect of lipophilicity on the organic nitrate antianginal drugs?
a) Increasing lipophilicity increases the activation of drug
b) Increasing lipophilicity decreases the activation of drug
c) It may increase or decrease the activation of drug
d) Increasing lipophilicity have no major effect on activity of drug
Q.4 The starting chemicals required for the synthesis of drug amyl nitrite?
a) Propanol
b) Butanol
c) Pentanol
d) Hexanol
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug amyl nitrite can be?
- Molecular weight = 117.15 gm/mol
- Physical appearance = colorless to yellowish liquid
- Boiling point = 97-99 oC
a) TFT
b) FFT
c) FFF
d) TTT
Q.6 Correct statements for the IUPAC nomenclatures of the drug are?
I. Amyl nitrite: (3-methylbutyl) nitrite
II. Flufenamic acid: 2-{[3-(Trifluoromethyl)phenyl]amino}benzoic acid
III. Ketorolac: (±)-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid
IV. Flubiprofen: (RS)-2-(2-fluorobiphenyl-4-yl)propanoic acid
a) I, II, III, IV
b) II, IV
c) II, III, IV
d) I, II
Q.7 Match the following drugs with their correct classifications-
| i. Amyl nitrite | A. Antianginal drug |
| ii. Tripelenamine | B. Diuretics |
| iii. Acetazolamide | C. Alkylating agent |
| iv. Thiotepa | D. H1-antagonist |
a) i-A, ii-C, iii-D, iv-B
b) i-A, ii-D, iii-B, iv-C
c) i-D, ii-B, iii-A, iv-C
d) i-D, ii-A, iii-C, iv-B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test
ANSWERS
1-c
2-a
3-d
4-c
5-d
6-a
7-b
REFERENCES
[1] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.