ATAZANAVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
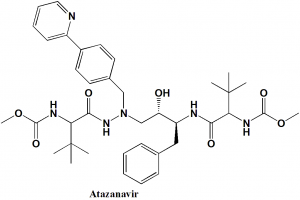
Atazanavir
IUPAC nomenclature
Methyl N-[(1S)-1-{[(2S,3S)-3-hydroxy-4-[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-N’-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate
Classification
Atazanavir is an HIV protease inhibitor.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 704.9 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | ~200°C |
| 4 | Solubility | 0.11 mg/L in water |
| 5 | Octanol/water partition coefficient | 4.5 |
| 6 | Presence of ring | Pyridine, phenyl |
| 7 | Number of chiral centers | 4 |
Mechanism of Action
Atazanavir binds with active site of HIV-1 protease enzyme and inhibits the virus specific processing of viral Gag and Gag-Pol polyprotiens. Due to this, there is formation of immature viral particles.
Atazanavir cannot be used against HIV-2 virus.
Structure Activity Relationship
- Isoamyl compound is potent against HIV-1 but have poor bioavailability.
- Substitution of isopropyl group with cyclohexyl improves oral biovailability but reduces antiviral activity by 10-fold. The antiviral activity is regained by replacing cyclohexylmethyl substituent of CGP-53820 with a biphenyl moiety and the two valine residues with tert-Leu. [1]
Method of synthesis
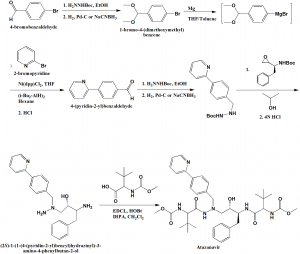
i. Reaction of Boc-(pyridin-2-yl)benzyl)hydrazine with epoxide, followed after depreotection by coupling with N-methoxycarbonyl-L-tert-leucine to get the Atazanavir.
ii. Required benzylhydrazine is prepared by coupling of 4-bromobenzaldehyde dimethyl acetal to 2-bromopyridine catalysed by 1,3-bis(diphenylphosphino)propane nickel (II) chloride and diisobutylaluminium hydride followed by acidic hydrolysis.
iii. 4-(pyridin-2-yl)benzaldehyde sp formed produces hydrazone with Boc-hydrazine in ethanol. This on hydrogenation on Pd-C catalyst or reduction with Sodium cyanoboroydride produces hydrazine building block.
iv. Opening of the N-Boc-protected epoxide with Boc-protected benzylhydrazine produces symmetrical N-Boc-protected aza-dipeptide mimetic.
v. The Boc-protected groups are cleaved off by acidic treatment and the product so obtained is coupled with N-methoxycarbonyl-L-tert-leucine to get Atazanavir. [2]
Therapeutic Uses
- Atazanavir for the treatment and prevention of HIV infection in the infants
Side Effects
Side effects of Atazanavir are:
- Nausea
- Headache
MCQs
Q.1 What can be the brand name for drug Atazanavir?
a) Reyataz
b) Cobaltaz
c) Levotaz
d) Pseudotaz
Q.2 Which amongst the following statements is/are incorrect related to the SAR of Atazanavir?
I. Isoamyl compound is potent against HIV-1 but have poor bioavailability.
II. Substitution of isopropyl group with cyclohexyl improves oral biovailability and increases antiviral activity by 10-fold.
a) I, II
b) II
c) I
d) Both I and II are correct
Q.3 Type of ring present in the structure of Atazanavir?
I. Phenyl
II. Cyclopentane
III. Pyridine
IV. Furan
a) I, III
b) II, III
c) I, IV
d) II, IV
Q.4 Side effects of drug Atzanavir is/are?
a) Nausea
b) Hair loss
c) Pale skin
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Atazanavir | A. 720 gm/mol |
| ii. Ritonavir | B. 704.9 gm/mol |
| iii. Phenobarbital | C. 300 gm/mol |
| iv. Temazepam | D. 232.23 gm/mol |
a) i-C, ii-A, iii-B, iv-D
b) i-D, ii-B, iii-C, iv-A
c) i-B, ii-A, iii-D, iv-C
d) i-A, ii-B, iii-C, iv-D
Q.6 An example of drug from class HIV protease inhibitor is?
a) Temazepam
b) Methotraxate
c) Atazanavir
d) Methotrexate
Q.7 Number of chiral centers present in the structure of atazanavir?
a) 4
b) 1
c) 2
d) 3
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-b
3-a
4-d
5-c
6-c
7-a