AURANOFIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
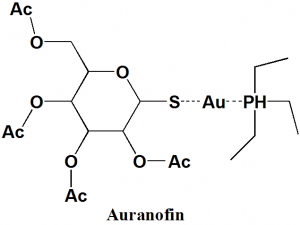
- Auranofin
IUPAC nomenclature
gold(1+);3,4,5-triacetyloxy-6-(acetyloxymethyl)oxane-2-thiolate;triethylphosphanium
Classification
- Synthetic Disease-Modifying Antirheumatic Drugs (DMARDs)
- Gold compounds
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 679.5 g/mol |
| 2 | Physical appearance | White crystalline powder |
| 3 | Melting point | 112-115oC |
| 4 | Solubility | Insoluble in water |
| 5 | Octanol/water partition coefficient | N/A |
| 6 | Presence of ring | Pyran |
| 7 | Number of chiral centers | 5 |
Mechanism of Action
- Auranofin inhibits the immune response by inhibiting kappab kinase and thioredoxin reductase enzymes.
- Decreases in the immune response decreases inflammation at the joint lining in the patients suffering from arthritis.
Structure Activity Relationship
General SAR for Gold compounds can be summarized as follows:
- Monovalent gold is more active than trivalent or colloidal gold.
- Attachment of the gold with the sulfur containing ligand is essential for the activity of the drug.
- Complexation of the gold ions with phosphine increases the bioavailability.
- Injectible gold compounds should be monocoordinated.
- Triethylphosphine gold compounds show the highest activity. [1]
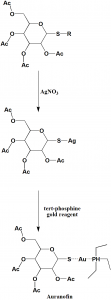
Method of synthesis
i. Reaction of thioether with silver nitrate or any other heavy metal salt to give silver salt of sugar thiol.
ii. On reacting the last with tert-phosphine gold reagent produces auranofin.[2]
Therapeutic Uses
Auranofin is used for:
- Treatment of patients having rheumatoid arthritis that did not respond to or cannot take other medication for the disease.
Side Effects
Side effects of Auranofin are:
- Abdominal cramping
- Nausea
- Loss of appetite
- Headache
- Heartburn
- Eye pain
- Eye redness
- Loss of hair
- Numbness
- Tingling in arms
- Stomatitis
- Pale skin
- Tiredness
- Easy bleeding
- Diarrhea
- Painful breathing
- Allergic reactions
- Skin rash
- Itching
MCQs
Q.1 “gold(1+);3,4,5-triacetyloxy-6-(acetyloxymethyl)oxane-2-thiolate;triethylphosphanium” is the IUPAC nomenclature of which drug?
a) Gold sodium thiomalate
b) Aurothioglucose
c) Auranofin
d) Oxymetazoline
Q.2 Appearance of auranofin is?
a) White crystalline solid
b) Yellow powder
c) Black amorphous solid
d) None of the above
Q.3 Match the following with correct classifications of the drugs.
| i. Auranofin | A. Non-selective adrenergic agonist |
| ii. Norepinephrin | B. Cholinestrase reactivator |
| iii. Pralidoxime | C. ß-blocker |
| iv. Carvedilol | D. DMARDs |
a) i-A, ii-D, iii-B, iv-C
b) i-D, ii-A, iii-B, iv-C
c) i-D, ii-C, iii-A, iv-B
d) i-C, ii-D, iii-A, iv-B
Q.4 Correct statements from below which are related with the mechanism of action of Auranofin can be??
I. It inhibits thioredoxin reductase enzyme
II. It activates thioridoxin reducatse enzyme
III. It decreases the immune response
IV. It increases the immune response
a) I, III
b) I, IV
c) II, III
d) II, IV
Q.5 Correct sequence for True and False for the given statements related with the SAR of Anti-inflammatory gold compounds can be?
- Monovalent gold is less active than trivalent or colloidal gold.
- Attachment of the gold with the sulfur containing ligand is essential for the activity of the drug.
- Complexation of the gold ions with phosphine decreases the bioavailability.
- Injectible gold compounds should be monocoordinated
a) TFFT
b) FFTT
c) FTFT
d) TTTT
Q.6 Silver salt of sugar thiol can produce Auranofin by reaction with?
a) Baeyer’s reagent
b) Silver nitrate
c) Tert-phosphine gold reagent
d) Lead acetate
Q.7 The drug Auranofin is mainly used for?
a) Treatment of Parkinson disease
b) For reversing the toxicity due to cholinergic drugs
c) Reducing pain due to arthritis
d) All of the above
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-c
2-a
3-b
4-a
5-c
6-c
7-c