AUROTHIOGLUCOSE Synthesis, SAR, MCQ, Structure, Chemical Properties and Therapeutic Uses
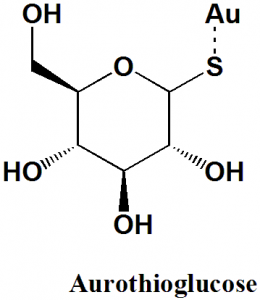
Aurothioglucose
IUPAC nomenclature
gold(1+);(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxane-2-thiolate
Classification
- Synthetic Disease-Modifying Antirheumatic Drugs (DMARDs)
- Gold compounds
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 392.18 g/mol |
| 2 | Physical appearance | Yellow crystals |
| 3 | Melting point | >107oC |
| 4 | Solubility | Soluble in water with decomposition; slightly soluble in propylene glycol; insoluble in acetone |
| 5 | Octanol/water partition coefficient | -2.50 (log Kow) |
| 6 | Presence of ring | Pyran |
| 7 | Number of chiral centers | 5 |
Mechanism of Action
- Aurothioglucose produces an inhibitory effect on the activity of adenyl cyclase in the human lymphocyte membranes and membranes of T and B lymphocyte subsets.
- This also results in the inhibition of the cyclases to induce mast cell degranulation and histamine release, enhancing respiratory burst effects, inducing and activating phagocytes, stimulating the action of resting macrophages, inducing neutrophil chemotaxis, -all of which are pro-inflammatory actions
Structure Activity Relationship
General SAR for Gold compounds DMARDs can be summarized as follows:
- Monovalent gold is more active than trivalent or colloidal gold.
- Attachment of the gold with the sulfur containing ligand is essential for the activity of the drug.
- Complexation of the gold ions with phosphine increases the bioavailability.
- Injectible gold compounds should be monocoordinated.
- Triethylphosphine gold compounds show the highest activity. [1]
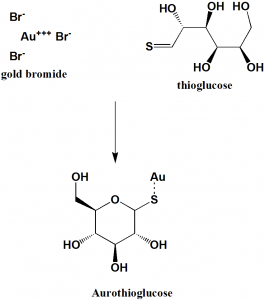
Method of synthesis
i. Addition of solution of gold bromide to the aqueous solution of thioglucose, containing sulfur dioxide.
ii. Precipitate can be obtained after heating solution and adding ethanol.[2]
Therapeutic Uses
Aurothioglucose is used for:
- Treatment of patients having rheumatoid arthritis that did not respond to or cannot take other medication for the disease.
Side Effects
Side effects of Aurothioglucose are:
- Changes in skin color
- Changes in heartbeat
- Cough
- Dizziness
- Fever
- Diarrhea
- Hives
- Nausea
- Vomiting
- Shortness of breath
- Inflammation of skin
- Weakness
- Respiratory tract infection
- Sore throat
- Spots on skin
- Allergic reactions
- Difficulty in breathing
- Easy bleeding
- Indigestion
- Itching
- Metallic taste
- Pale skin and eyes
MCQs
Q.1 Mechanism of action of Aurothioglucose is due to?
a) Inhibitting effect on the activity of adenyl cyclase in the human lymphocyte membrane and membrane of T and B lymphocytes
b) Inhibition of Acetylcholinestrase which hen results in the decrease in the level of acetylcholine at the synapse
c) Alkylation of the genetic material which help in killing the neoplastic cells
d) None of the above
Q.2 Therapeutic use of drug Aurothioglucose is/are?
a) Treatment of Rheumatoid arthritis
b) Treatment of malignant tumors
c) As an anesthetic agent
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of Gold compounds DMARDs?
I. Monovalent gold is less active than trivalent or colloidal gold.
II. Attachment of the gold with the sulfur containing ligand is essential for the activity of the drug.
III. Complexation of the gold ions with phosphine decreases the bioavailability.
IV. Injectible gold compounds should be monocoordinated.
a) I, II, III, IV
b) II, IV
c) I, III, IV
d) II, III
Q.4 Aurothioglucose can be synthesized by adding gold bromide to?
a) Dihydrofuran
b) Glycopyrrolate
c) Thioglucose
d) Both a) and c)
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Aurothioglucose?
- Molecular weight: 392.18 gm/mol
- Physical appearance: Yellow crystals
- Melting point: 78-820C
- Octanol/water partition coefficient: 7.9
a) TFFT
b) FFTF
c) TTFF
d) FFFF
Q.6 Correct statements for the IUPAC nomenclatures of the drugs are?
I. Aurothioglucose: gold(1+);(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxane-2-thiolate
II. Carboplatin: (SP-4-2)-diamminedichloroplatinum(II)
III. Lopinavir: 1,4-dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]-anthracene-9,10-dione.
IV. Chloraquine: 2-amino-1H-purine-6(7H)-thione
a) I, II
b) II, III, IV
c) I, IV
d) I, II, III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Aurothioglucose | A. Benzodiazepine sedative hypnotic |
| ii. Quazepam | B. DMARDs |
| iii. Bisoprolol | C. Cholinergic agonist |
| iv. Acetylcholine | D. ß-adrenergic antagonist |
a) i-A, ii-D, iii-B, iv-C
b) i-B, ii-A, iii-D, iv-C
c) i-B, ii-C, iii-A, iv-D
d) i-A, ii-B, iii-C, iv-D
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-a
3-b
4-c
5-c
6-a
7-b