CEVIMELINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
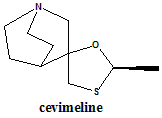
Cevimeline
IUPAC nomenclature
(2R,2R)-2′-Methylspiro[4-azabicyclo[2.2.2]octane-2,5′-[1,3]oxathiolane].
Classification
Cevimeline is a cholinergic (muscarinic) agonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 199.32 g/mol |
| 2 | Physical appearance | White to off white crystalline powder |
| 3 | Melting point | 201-203°C. |
| 4 | Solubility | 4.1X10+4 mg/L at 25 °C in water |
| 5 | Presence of ring | Oxathiolane and quinuclidine |
| 6 | Number of chiral centers | 3 |
Mechanism of Action
- Cevimeline binds with muscarinic M1 and M3 receptors.
- Due to binding with M1 receptors, there is increase in the secretion from the exocrine glands such as salivary glands.
- Cevimeline binds with M3 receptors and activation of it results in stimulation of the salivary glands, and also, smooth muscle contraction and grandular secretion increases.
- These help in relieving from the symptoms of dry mouth.
Structure Activity Relationship
- When the nitrogen of the quaternary ammonium group is replaced with arsenic, phosphorous, sulfur or selenium, their activity decreases.
- It is necessary for the atom present at the nitrogen position to have a positive charge to have muscarinic activity.
- When all three methyl groups are replaced by larger alkyl groups at the quaternary nitrogen, drugs become inactive as agonist.
- When all the methyl groups associated with the quaternary nitrogen atom are replaced by ethyl groups, the compound becomes cholinergic antagonist.
- Replacement of just one methyl group associated with quaternary nitrogen with an ethyl or a propyl group gives the compound which is having lesser activity than acetylcholine.
- Substitution of the methyl groups associated with quaternary nitrogen with hydrogen atoms also leads to diminishing of the muscarinic activity.
- At the ethylene bridge, synthesis of acetic acid esters of quaternary ammonium alcohols of greater length than choline led to a series of compounds which are having lesser activity as the length of the chain increases.
- There should be no more than 5 atoms between nitrogen and terminal hydrogen to have the maximum muscarinic activity.
- Replacement of the ethylene bridge by the alkyl groups larger than methyl groups decreases the activity.
- The R-(-) isomer is 20 time less potent.
- For the maximum potency, the alkyl groups at the nitrogen atoms should not be larger than methyl groups.
- Presence of oxygen in an ester form increases the activity.
- For maximum activity, there should be two- carbon atoms between the nitrogen and oxygen atom. [1]
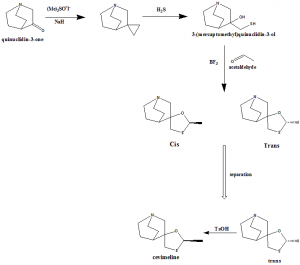
Method of synthesis
i. Reaction of quinuclidin-3-one with trimethylsulfoxonium iodide and NaH in DMSO to give epoxide.
ii. The epoxide is opened with SH2 in NaOH/water, yielding 3-hydroxy-3-(sulfanylmethyl)quinuclidine.
iii. Cyclization of latter compound with acetaldehyde catalyzed by boron tridluoride ethearate or by SnCl4, POCl3, H3PO4 or p-toluenesulfonic acid gives mixture of two diastereomeric spiroacemates.
iv. Mixture is separated through fractional recrystallization in acetone to get Cevimeline.
Therapeutic Uses
Cevimeline is used for treatment of:
- Symptoms of dry mouth
Side Effects
Side effects of cevimeline are:
- Vision problems
- Diarrhea
- Weakness
- Dizziness
- Frequent micturition
- Flushing
- Chills
- Runny nose
- Nausea
- Sweating
MCQs
Q.1 “(2R,2R)-2′-Methylspiro[4-azabicyclo[2.2.2]octane-2,5′-[1,3]oxathiolane].” is the IUPAC nomenclature of which drug?
a) Acetylcholine
b) Cevimeline
c) Trospium chloride
d) Carvedilol
Q.2 Molecular weight of Cevimeline is?
a) 146.21 gm/mol
b) 199.32 gm/mol
c) 428 gm/mol
d) 406.5 gm/mol
Q.3 Match the following with correct classifications of the drugs.
| i. Cavedilol | A. Antispasmodic agent |
| ii. Trospium chloride | B. Ach mimetic |
| iii. Cevimeline | C. Mixed α/ß blocker |
| iv. Metocurine | D. Nicotinic antagonist |
a) i-,B ii-,C iii-A, iv-D
b) i-D, ii-A, iii-C, iv-B
c) i-C, ii-A, iii-B, iv-D
d) i-B, ii-A, iii-C, iv-A
Q.4 Cevimeline shows its action through binding with?
a) M1, M2 receptors
b) M1, M5 receptors
c) M1 to M5 receptors
d) M1, M3 receptors
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug Cevimeline.
- When the nitrogen of the quaternary ammonium group is replaced with arsenic, phosphorous, sulfur or selenium, their activity Increases.
- It is necessary for the atom present at the nitrogen position to have a Negative charge to have muscarinic activity.
- When all three methyl groups are replaced by larger alkyl groups at the quaternary nitrogen, drug becomes inactive as agonist.
- When all the methyl groups associated with the quaternary nitrogen atom are replaced by ethyl groups, the compound becomes cholinergic antagonist.
a) TTFF
b) FTFT
c) TFFT
d) FFTF
Q.6 Starting materials for the synthesis of cevimeline can be?
a) Amphetamine and Atropine
b) Pyrrolidine, Quiniclidine
c) Quiniclidin-3-one, trimethylsulfoxonium iodide and NaH
d) Pyrrolidine and trimethylsulfoxonium iodide
Q.7 The drug Cevimeline is mainly used for?
a) Symptoms of dry mouth
b) Alzheimer’s disease
c) Malaria
d) None of the above
FREE GPAT online Test: Participate: Click Here
ANSWERS
1-b
2-b
3-c
4-d
5-a
6-c
7-a
REFERENCES
[1] Lemke TL, Williams DA, Foye WO. Principles of medicinal chemistry. Williams & Wilkins; 2017, 320.