CLEMASTINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
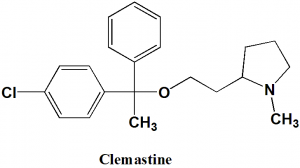
Clemastine
IUPAC nomenclature
(2R)-2-{2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine
Classification
- H1-receptor antihistamine
- Ethanolamine ether antihistamine
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 343.9 g/mol |
| 2 | Physical appearance | Solid |
| 3 | Melting point | 178oC |
| 4 | Solubility | Hydrogen fumarate formulation is soluble |
| 5 | Octanol/water partition coefficient | 5.2 |
| 6 | Presence of ring | Pyrrolidine, phenyl |
| 7 | Number of chiral centers | 2 |
Mechanism of Action
i. Clemastine binds with Histamine H1 receptor and produces antagonistic effects.
ii. This results in blockage of action of endogenous histamine
iii. Temporary relief of negative symptoms produced due to histamine
Structure Activity Relationship
Structure activity of ethanolamine ethers can be summarized as:
- 8-chlorotheophyllinate salt of diphenhydramine is used for treatment of motion sickness
- Compounds with p-Cl-Ph and 2-pyridyl aryl groups are carboxamine, which is a potent anti-histamine drug.
- Substitution of the methyl group at the carbon alpha to the ether function gives a related compound known as doxylamine.
- An additional carbon atom between oxygen and nitrogen produces clemastine which has lower sedative properties.
- In setastine, alkyl amine substituent is incorporated into seven-membered hexahydroazepine ring, having lower sedative properties.
- With increase in alkyl group size at C-2’, there is decrease in antihistaminic activity and increases in anticholinergic activity..
- Introduction of alkyl substituents at C-4’results in decrease in anticholinergic activity and increase in antihistaminic activity.[1]
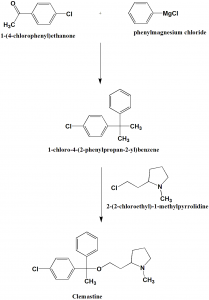
Method of synthesis
i. Reaction of 4-chloroactophenone with phenylmagnesium bromide to give 1-(4-chlorophenyl)-1-phenylethanol.
ii. The last is reacted with 2-(2-chlorethyl)-1-methylpyrrolidine using sodium amide as base to give clemastine [2]
Medicinal Uses
Clemastine is used for treatment of:
- Sneezing
- Watery eyes
- Runny nose
- Skin rash
- Hay fever
- Itching
- Cold symptoms
- Allergy symptoms
Side Effects
Side effects of Clemastine are:
- Drowsiness
- Blurred vision
- Restlessness
- Dizziness
- Headache
- Stomach upset
- Constipation
- Dry mouth, nose and throat
- Difficulty in breathing
- Mental or mood changes
- Hallucinations
- Allergic reactions
- Seizures
MCQs
Q.1 Which of the following statements related with the physical properties of drug clemastine?
Molecular weight: 343.9 gm/mol
Physical appearance: Oily liquid
III. Melting point: 178oC
Octanol/water partition coefficient: 5.2
a) I, II, IV
b) II, IV
c) III, IV
d) I, V
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Clemastine | A. gold(1+);3,4,5-triacetyloxy-6-(acetyloxymethyl)oxane-2-thiolate;triethylphosphanium |
| ii. Auranofin | B. 4-hydroxy-2-methyl-N-(5-methyl-1,3-thiazol-2-yl)-1,1-dioxo-1λ6,2-benzothiazine-3-carboxamide |
| iii. Meloxicam | C. (2R)-2-{2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl}-1-methylpyrrolidine |
| iv. Ketorolac | D. (±)-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid |
a. i-A, ii-B, iii-D, iv-C
b. i-B, ii-D, iii-C, iv-A
c. i-C, ii-A, iii-B, iv-D
d. i-B, ii-A, iii-C, iv-D
Q.3 Mechanism of action of Clemastine includes?
I. It binds with Histamine H1 receptor and produces antagonistic effects.
II. Permanent relief in negative symptoms produced due to histamine
III. Binding with alpha-adrenergic receptors
a) I, II
b) II, III
c) I, III
d) III
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Clemastine: Ethanolamine ether antihistamine drug
- Naproxen: NSAID
- Diclofenac: NSAID
- Desflurane: Inhalational anesthetics
a) TTTT
b) FTTF
c) FTTF
d) TTFF
Q.5 8-Chlorotheophyllinate salt of diphenhydramine is used for treatment of?
a) Motion sickness
b) Bradyarrhythmia
c) Pain
d) All of the above
Q.6 The correct sequence for the steps for synthesis of drug clemastine from 4-chloroacetophenone can be?
I. Reaction with phenylmagnesium bromide
II. Reaction with 2-(2-chloroethyl)-1-methylpyrrolidine
III. Reaction with sodium amide
a) I – II – III
b) I – III – II
c) III – II – I
d) II – I – III
Q.7 Side effect of drug clemastine?
a) Sneezing
b) Skin rash
c) Blurred vision
d) All of the above
List of Successful GPATINDIAN Candidates
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-a
2-c
3-a
4-a
5-a
6-b
7-c
REFERENCES
[1] Lemke TL, Williams DA, editors. Foye’s principles of medicinal chemistry. Lippincott Williams & Wilkins; 2012 Jan 24. [2] Vardanyan R, Hruby V. Synthesis of essential drugs. Elsevier; 2006 Mar 10.