CLOZAPINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
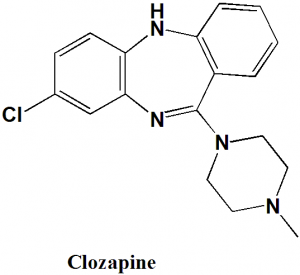
Clozapine
IUPAC nomenclature
8-Chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine.
Classification
Clozapine is an atypical antipsychotic drug.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 326.8 g/mol |
| 2 | Physical appearance | Yellow crystals from acetone-petroleum ether |
| 3 | Melting point | 183.5°C |
| 4 | Solubility | In water, 29.15 mg/L at 25 °C |
| 5 | Octanol/water partition coefficient | 3.23 |
| 6 | Presence of ring | Piperazine ring |
| 7 | Number of chiral centers | Not present |
Mechanism of Action
- Antagonistic effects at D2 receptors in the mesolimbic pathway which relieves positive symptoms.
- Antagonistic effects at 5-HT2A receptors in the frontal cortex which alleviates negative symptoms.
Structure Activity Relationship
Structure activity relationship of phenothiazine like compounds can be described as follows:
- Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
- Optimal neuroleptic activity occurs when the ring A substituent is in the 2nd-position.
- A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
- A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
- Hydroxyethylpiperazine side chain phenothiazines displays more favorable Van der Waal’s interactions with ring A than simple piperazines.
- In the thioxanthene and xanthenes containing ring systems, the cis forms are more potent neuroleptics than the trans isomers.
- Phenothiazine analogues having the presence of exolytic double bond are more potent than the corresponding compounds lacking the exolytic double bonds. [1]
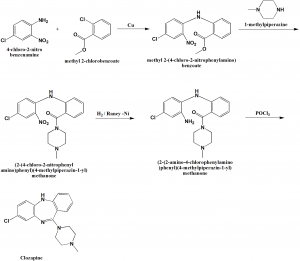
Method of synthesis
i. 4-chloro-2-nitroaniline is acylated in the presence of copper filings by the o-chlorobenzoic acid methyl ester to form corresponsing diphenylamine.’the ester group in the resulting polyfunctional diphenylamine is transformed into amide by reacting with N-methyl piperazine.
ii. Nitro group of the resulting amide undergoes reduction in the presence of Raney nickel into an amine by hydrogen.
iii. Reacting the product with phosphorus oxychloride yields in heterocyclization into the desired clozapine . [2]
Therapeutic Uses
Clozapine is used for:
- Treatment of schizophrenia
- Reducing nervousness
- Decreasing hallucinations
Side Effects
Side effects of Clozapine are:
- Dizziness
- Drowsiness
- Lightheadedness
- Drooling
- Constipation
- Weight gain
- Rise in blood sugar level
- Rise in cholesterol level
MCQs
Q.1. Pick the correct statement with respect to drug clozapine?
a) It antagonizes D1 dopaminergic receptors
b) It antagonizes D2 dopaminergic receptors
c) It antagonizes α-adrenoceptors
d) It antagonizes ß-adrenoceptors
Q.2 Therapeutic use of drug clozapine is/are?
a) Treatment of Schizophrenia
b) Reducing mervousness
c) Decreasing hallucinations
d) All of the above
Q.3 Which amongst the following are the correct statements with respect to the SAR of drug Clozapine?
I. Tilting of side chain towards ring A grants favorable Vander Waal’s interaction of the side chain. This interaction decides the potency of the drug towards the dopamine receptors.
II. Optimal neuroleptic activity occurs when the ring A substituent is in the 2nd-position.
III. A trifluoromethyl substituent provides a greater number of favorable Van der Waal’s contacts with the side chain than the chlorine substituent. Thus, phenothiazne with trifluoromethyl substituents are more potent than those with chlorine substituent.
IV. A piperazine side chain provides more Van der Waal’s contacts with 2-substituent than the alkylamino side chain. Thus, piperizine phenothiazine are more potent in antischizophrenic effects than alkylamino phenothiazines.
a) I, III, IV
b) II, IV
c) I, II, III, IV
d) III, IV
Q.4 Number of chiral carbons present in the structure of clozapine?
a) 0
b) 2
c) 3
d) 4
Q.5 Correct sequence for the True/False for the physiochemical properties of the drug Clozapine?
I. Molecular weight is 326.8 gm/mol
II. It is present in viscous liquid form
III. Piperizine ring is not present
a) FTF
b) FFT
c) FTT
d) TFF
Q.6 Correct statements for the IUPAC nomenclatures of the are?
I. Clozapine: 7-chloro-5-(2-fluorophenyl)-1-(2,2,2-trifluoroethyl)-3H-1,4-benzodiazepine-2-thione
II. Loxapine: 8-chloro-6-(4-methylpiperazin-1-yl)benzo[b][1,4]benzoxazepine
III. Temazopam: 7-Chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-1,4-benzodiazepin-2-one
IV. Quazepam: 7-chloro-1-methyl-5-phenyl-1,5-benzodiazepine-2,4-dione
a) I, II
b) I, IV
c) II, III
d) III, IV
Q.7 Match the following drugs with their correct classifications-
| i. Clozapine | A. Atypical antipsychotic drug |
| ii. Tolazoline | B. Hydantoin anticonvulsant |
| iii. Esmolol | C. α-adrenergic blocker |
| iv. Phenytoin | D. ß-blocker |
a) i-C, ii-A, iii-D, iv-B
b) i-A, ii-C, iii-D, iv-B
c) i-B, ii-C, iii-D, iv-A
d) i-D, ii-C, iii-A, iv-B
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-d
3-c
4-a
5-d
6-c
7-b