Concept of diffusion: Applications of diffusion principles and MCQs for GPAT, NIPER, Pharmacist and Drug Inspector exam
APPLICATIONS OF DIFFUSION PRINCIPLES:
- Drug Absorption by Passive Diffusion – Passive diffusion is the major trans-membrane transport process for most drugs. The driving force for passive diffusion is the difference in drug concentrations on either side of the cell membrane. This process is passive because no external energy is expended. According to Fick’s law of diffusion, drug molecules diffuse from a region of high drug concentration to a region of low drug concentration:
dQ/dt = (DAK/h) X (CGI – CP) (1)
where dQ/dt is the rate of diffusion, D the diffusion coefficient, K the partition coefficient, A the surface area of membrane, h the membrane thickness and C GI – Cp the difference between the concentrations of drug in the GI tract and in the plasma. As shown by Fick’s law of diffusion, the rate of passive diffusion of drugs depends on the lipid solubility of the drug (K), surface area (A) and the thickness of the membrane (h).
- Drug Absorption by Facilitated Diffusion – Facilitated diffusion is a non energy requiring, carrier-mediated transport system in which the drug moves along a concentration gradient (i.e. moves from a region of high drug concentration to a region of low drug concentration). The main characteristics of facilitated diffusion is that transport occurs down a concentration gradient, membrane permeability exceeds that predicted from partition coefficients, transport is saturable and competition occurs between isomers. Facilitated diffusion has been used to explain the cellular uptake of sugars and amino acids.
- Percutaneous or Transdermal Absorption – The percutaneous absorption or the transdermal delivery of a drug occurs in the following manner. Initially a topically applied drug is absorbed into the stratum corneum and diffuses through that layer of skin into the epidermis and then into the dermis where drug molecules reach the capillaries and enter the circulatory system. Diffusion through the stratum corneum is the rate-determining step unless skin perfusion is decreased. In the latter case, diffusion is controlled by the transfer of drug molecules into capillaries rather than by the diffusion process previously explained. Drug diffusion may be explained by the following equation:
J = Km X Dm X Cs/l (2)
where J is flux, K m the partition coefficient, Dm the diffusion constant under specific conditions such as temperature and hydration, Cs the concentration gradient and l the length or thickness of the stratum corneum.
- Ostwald Ripening in Emulsions – The principal mechanism for irreversible droplet growth, called Ostwald ripening, in submicronic emulsions (particularly in the case of perfluorocarbon emulsions) during storage is molecular diffusion. Molecular diffusion involves the transfer of individual molecules from the smaller droplets, where the chemical potential is higher due to the higher curvatures of the particles (Kelvin effect), through the continuous aqueous phase to join larger ones, resulting in the irreversible increase of droplet size over time. Molecular diffusion in emulsions can be effectively slowed down by including a small amount of a component with lesser water
solubility in the dispersed phase. - Bioadhesion and Mucoadhesion – Bioadhesion is defined as the attachment of synthetic or biological macromolecules to a biological tissue and mucoadhesion is a special case of bioadhesion where the biological tissue is an epithelium covered by mucus. The mechanistic processes involved in mucoadhesion are as follows:
- Wetting and swelling of the polymer for intimate contact with the biological tissue.
- Interpenetration of the bioadhesive polymer chains and entanglement of polymer and mucin chains.
- Formation of weak chemical bonds between entangled chains.
The penetration of polymer chains into the mucus depends on concentration gradients and diffusion coefficients. The driving force for inter diffusion is the concentration gradient across the interface. It is believed that for an effective adhesion bond, the interpenetration of the polymer chain should be in the range of 0.2–0.5 mm. It is also possible to estimate the penetration depth (l) by
l = (tDb)1/2 (3)
where t is the time of contact and Db is the diffusion coefficient of the bioadhesive material in the mucus.
- In Vitro Permeation Studies – For assessing the absorption potential of drug candidates or conducting studies evaluating correlations between drug structure and transport, in vitro models may provide the best approach. Numerous investigators have employed diffusion chambers of various designs (e.g. Using chambers, Sweetana–Grass diffusion cells) to evaluate the permeation properties of drug candidates. These systems offer a distinct advantage that drug solutions may be added to the donor compartment under various conditions (e.g. varying concentrations, pH, excipients). Samples are withdrawn periodically from a receiver compartment and analyzed for drug content to obtain a true measure of permeation across the cellular barrier. In establishing procedures for these diffusion cell studies, the underlying muscle layer can be teased away and only the intact mucosal cell layer mounted in the diffusion chamber.
- Osmotically Controlled Delivery Systems – The process of diffusion of a solvent through a semipermeable membrane from a less concentrated solution into a more concentrated solution is called osmosis. Basically, when two different concentrations are separated by a semipermeable membrane, osmotic pressure builds up on the higher-concentration side. Several attempts have been made to use osmotic
pressure as a driving force to deliver drugs. Osmotic pump tablets were developed by compressing drug and osmogen (NaCl) into a hard tablet, followed by coating the tablet with a semipermeable membrane (e.g. cellulose acetate) and then drilling an orifice in the coating by a laser.
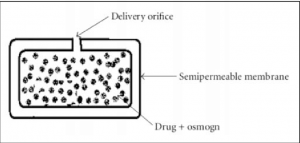
Fig 1 – An elementary osmotic pump (taken from elementary osmotic pump/download scientific diagram)
Upon contact with water, the semipermeable membrane of an osmotic pump tablet absorbs water, and water diffuses through the membrane and dissolves water soluble substances, resulting in a concentrated solution and high osmotic pressure inside the membrane. This leads to drawing more water across the membrane. The rate of drug delivered through the orifice (dM/dt) is then given by
dM/dt = dQ/dt X C (4)
where dQ/dt is the flow rate of water across the membrane and C is the drug concentration inside the membrane.
The drug release from such delivery systems follows nearly zero-order kinetics. Commercial examples of the elementary osmotic pump system are Accutrim (phenylpropanolamine HCl), Efidac 24 (chlorpheniramine) and Sudafed 24 (pseudoephedrine).
Multiple choice questions:
1.Passive diffusion is the major trans-membrane transport process for most drugs.
a)true
b)false
2.According to Fick’s law of diffusion, drug molecules diffuse from a region of ___drug concentration to a region of ____ drug concentration.
a)high,low
b)low,high
c)high,high
d)low,low
3.dQ/dt = (DAK/h) X (CGI – CP) In this equation D is
a)rate of diffusion
b)diffusion coefficient
c)partition coefficient
d)surface area of membrane
4.dQ/dt = (DAK/h) X (CGI – CP) In this equation K is
a)rate of diffusion
b)diffusion coefficient
c)partition coefficient
d)surface area of membrane
5.According to Fick’s law of diffusion, the rate of passive diffusion of drugs depends on
a)lipid solubility of the drug (K)
b)surface area (A)
c)thickness of the membrane (h)
d)all of these
6.Facilitated diffusion is a non energy requiring, carrier-mediated transport system in which the drug moves along
a)concentration gradient
b)moves from a region of high drug concentration to a region of low drug concentration
c)both of these
d)only b
7.Facilitated diffusion has been used to explain the cellular uptake of
a)sugars
b)amino acids
c)lipids
d)a and b
8.Initially a topically applied drug is absorbed into the
a)stratum corneum
b)epidermis
c)dermis
d)circulatory system
9.Drug diffusion through Percutaneous or Transdermal Absorption may be explained by which of the following equation?
a)dQ/dt = (DAK/h) X (CGI – CP)
b)J = Km X Dm X Cs/l
c)l = (tDb)1/2
d)dM/dt = dQ/dt X C
10.The attachment of synthetic or biological macromolecules to a biological tissue is called
a)Bioadhesion
b)mucoadhesion
c)both of these
d)none of these
11.The mechanistic processes involved in mucoadhesion is/are as follows:
a)Wetting and swelling of the polymer for intimate contact with the biological tissue
b)Interpenetration of the bioadhesive polymer chains and entanglement of polymer and mucin chains
c)Formation of weak chemical bonds between entangled chains
d)all of these
12.For an effective adhesion bond, the interpenetration of the polymer chain should be in the range of
a)0.2–0.5 mm
b)0.1–0.5 mm
c)0.2–0.4 mm
d)0.25–0.5 mm
13.The process of diffusion of a solvent through a semipermeable membrane from a less concentrated solution into a more concentrated solution is called
a)diffusion
b)osmosis
c)dissolution
d)solubilisation
14.An elementary osmotic pump consist of
a)delivery orifice
b)semipermeable membrane
c)drug + osmogen
d)all of these
15.The drug release from Osmotically Controlled Deliver System follows which of the following kinetics?
a)zero-order kinetics
b)first order kinetics
c)secondnd order kinetics
d)none of these
Solutions:
- a)true
- a)high,low
- b)diffusion coefficient
- c)partition coefficient
- d)all of these
- c)both of these
- d)a and b
- a)stratum corneum
- b)J = Km X Dm X Cs/l
- a)Bioadhesion
- d)all of these
- a)0.2–0.5 mm
- b)osmosis
- d)all of these
- a)zero-order kinetics
References:
- Gaurav K. Jain Theory and Practice of Physical Pharmacy, 1st edition 2012 Elsevier, page no. 271-274.
List of Successful GPATINDIAN CANDIDATES
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
Participate in CSIR NET JRF Mock Test