DARUNAVIR Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
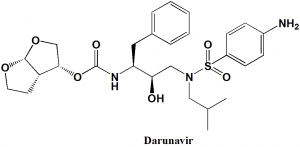
Darunavir
IUPAC nomenclature
[(1R,5S,6R)-2,8-dioxabicyclo[3.3.0]oct-6-yl] N-[(2S,3R)-4- [(4-aminophenyl)sulfonyl- (2-methylpropyl)amino]-3-hydroxy-1-phenyl- butan-2-yl] carbamateClassification
Darunavir is an HIV protease inhibitor.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 547.7 g/mol |
| 2 | Physical appearance | White amorphous solid |
| 3 | Melting point | 74°C |
| 4 | Solubility | 8.7 mg/L in water |
| 5 | Octanol/water partition coefficient | 1.8 |
| 6 | Presence of ring | Furan, phenyl |
| 7 | Number of chiral centers | 4 |
Mechanism of Action
Darunavir binds with HIV-1 protease enzyme and prevents the HIV replication through halting the dimerization and catalytic activity of HIV-1 protease. It specifically inhibits the cleavage of HIV encoded Gag-Pol proteins in cells, which ceases the generation of the mature virus particle and thus checks the spread of infection.
Structure Activity Relationship
- Darunavir have activity against multi-drug resistant strains of HIV-1.
- It is having high affinity binding to the N-H groups of P2 pocket of the HIV-1 protease.
- The large portion of the drug rest on the substrate envelope. [1]
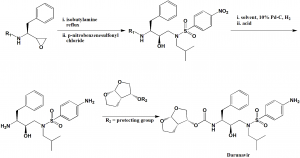
Method of synthesis
i. Isobutylamine is reacted with protected (2S,3S)-1,2-epoxy-3-amino-4-phenylbutane.
ii. The reaction is followed by reaction with p-nitrobenzenesulfonyl chloride in the presence of the base to get protected N-((2R,3S)-3-amino-2hydroxy-4-phenylbutyl)-/V-isobutyl-4-nitro-benzenesulfonamide.
iii. Reducing the formed compound and hydrolyzing.
iv. Coupling of the compound with (3R, 3aS, 6aR)-hexahydrofuro[2,3-6]furan-3-ol derivative to get darunavir. [2]
Therapeutic Uses
- Darunavir is used for the treatment and prevention of HIV infection.
Side Effects
Side effects of Darunavir are:
- Nausea
- Headache
- Vomiting
- Stomach pain
MCQs
Q.1 What can be the brand name for drug Darunavir?
a) Otogesic
b) Prezista
c) Nupercaine
d) Bendzon
Q.2 Therapeutic use of Darunavir is?
a) Treatment of HIV
b) Treatment of Anxiety
c) Treatment of diabetes
d) Treatment of cancer
Q.3 Type of ring present in the structure of Darunavir?
I. Phenyl
II. Purine
III. Pyridine
IV. Furan
a) I, III
b) II, III
c) I, IV
d) II, IV
Q.4 Side effects of drug Darunavir is/are?
a) Nausea
b) Headache
c) Vomiting
d) All of the above
Q.5 Match the following drugs with their correct molecular weights-
| i. Darunavir | A. 312 gm/mol |
| ii. Oseltamivir | B. 332 gm/mol |
| iii. Zanamivir | C. 547.7 gm/mol |
| iv. Ribavirin | D.244 gm/mol |
a) i-C, ii-A, iii-B, iv-D
b) i-D, ii-B, iii-C, iv-A
c) i-B, ii-A, iii-D, iv-C
d) i-A, ii-B, iii-C, iv-D
Q.6 An example of drug from class HIV protease inhibitor is?
a) Amprenavir
b) Darunavir
c) Atazanavir
d) All of the above
Q.7 Number of chiral centers present in the structure of darunavir?
a) 4
b) 1
c) 2
d) 3
Participate in Online FREE GPAT TEST: CLICK HERE
Participate in Online FREE Pharmacist TEST: CLICK HERE
Participate in Online FREE Drug Inspector TEST: CLICK HERE
ANSWERS
1-b
2-a
3-c
4-d
5-a
6-d
7-a