DAUNORUBICIN Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
Daunorubicin
IUPAC nomenclature
(8S,10S)-8-Acetyl-10-[(2S,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,8,11-trihydroxy-1-methoxy-9,10-dihydro-7H-tetracene-5,12-dione.

Classification
Duanorubicin falls under the category of Anthracyclic antibiotic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 527.5 g/mol |
| 2 | Appearance | Thin red needle form |
| 3 | Melting point | 208.5°C |
| 4 | Solubility | Soluble in methanol, chloroform and benzene |
| 5 | Octanol water partition coefficient | 1.83 |
| 6 | Presence of ring | dihydroxyanthraquinone ring |
Mechanism of Action
i. Duanorubicin forms complexes with DNA by intercalation between base pairs.
ii. The drug then stabilizes DNA-topoisomerase II and thus topoisomerase activity is inhibited.
iii. Relegation of the DNA is prevented.
iv. Cell death. [2]
Structural Activity Relationship
- Substitution at 2nd position decreases the biological activity of drug.
- Presence of any substituent at R2 position also decreases the biological activity of drug.
- Biological activity can be increased by substitution at 3rd
- 8th position has direct relationship with the biological activity of drug and thus, substitution at 8th position can increase the biological activity of drug.
- Substitution at 1st and 7th position will have negative impact on the biological activity of the drug.[3]
Methods of Synthesis
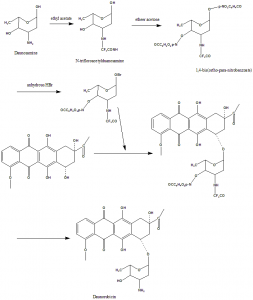
Danorubicin can be obtained from cultures of Streptomyces caeruleorubidus and Streptomyces peucetius.
TOTAL SYNTHESIS:
i. N-trifloroacetylduanosamine was obtained from duanosamine hydrochloride by ethyl acetate.
ii. The 1,4-bis(o-p—nitro-benzoate was obtained from ether acetone and suspended in CH2Cl2 at freezing temperature.
iii. It was saturated with Anhydrous HBr to give clear solution.
iv. P-nitrobenzoic acid was precipitated from the above clear solution.
v. Filteration and evaporation afforded the residual 1-bromo sugar.
vi. When the product was identified from silica gel in ethyl acetate-benzene-methanol, the impurities were separated through column chromatography.
vii. Recrystallization with 95% ethanol gives productwith 50% yield. [4]
Therapeutic Uses
Doxorubicin is used for the treatment of :
- Acute Myelogenous leukemia
- Acute lymphoblastic leukemia
- Acute promyelocytic leukemia
Side Effects
- Common side effects includes low blood counts, pain, nausea and vomiting.
- Some people may suffer from side effects like diarrhea, infertility, skin darkening and discoloration of nail beds.
MCQs
Q.1 Match the following with correct Trade names of the drugs-
| i. Daunorubicin | A. Mustine |
| ii. Mechlorethamine | B. Cirubidine |
| iii. Ifosfamide | C. Dacarin |
| iv. Dacarbazine | D. Holoxan |
a) i-D, ii-A, iii-B, iv-C
b) i-B, ii-A, iii-D, iv-C
c) i-C, ii-A, iii-D, iv-B
d) i-D, ii-B, iii-A, iv-C
Q.2 How many statements below are true with respect to the SAR of the drug Daunorubicin?
- Substitution at 2nd position Increases the biological activity of drug.
- Presence of any substituent at R2 position also decreases the biological activity of drug.
- Biological activity can be increased by substitution at 3rd
- 8th position has direct relationship with the biological activity of drug and thus, substitution at 8th position can increase the biological activity of drug.
a) 1
b) 2
c) 3
d) 4
Q.3 The drug Daunorubicin is found in which form?
a) Thin reddish needles form
b) Yellow prism form
c) Brown poder form
d) None of the above
Q.4 The correct statement/s with respect to the method of synthesis of the drug Daunorubicin is/are?
a) Daunorubicin is obtained from cultures of Streptomyces peucetius
b) Daunorubicin is obtained from cultures of Streptomyces caeruleorubidus
c) Both a) and b)
d) None of the above
Q.5 Which amongst the following is not a therapeutic use of drug Daunorubicin?
a) AML
b) Hair loss
c) ALL
d) APL
Q.6 The correct classification of the drug Daunorubicin can be?
a) Triazine
b) Antibiotics
c) Anti folate agent
d) Pyrimidine antagonist
Q.7 How many number of rings are found in the chemical structure of the drug Daubnorubicin?
a) 5
b) 4
c) 3
d) 2
ANSWERS
1-b
2-c
3-a
4-d
5-b
6-b
7-a