MITOXANTRON Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
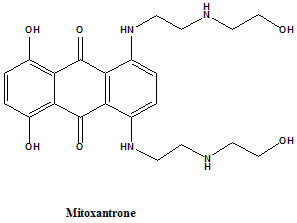
Mitoxantron
IUPAC nomenclature
1,4-dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]-anthracene-9,10-dione.
Classification
Mitoxantron falls under the category of antibiotic Antineoplastic cytotoxic drug. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 444.5g/mol |
| 2 | Appearance | Present in liquid state.
Can be obtained as blue-black solid form from water ethanol. |
| 3 | Melting point | 203-205 °C |
| 4 | Solubility | Slightly soluble in methyl alcohol, sparingly soluble in water. |
| 5 | Octanol water partition coefficient | -3.1 |
| 6 | Presence of ring | Dihydroxyanthracene ring |
Mechanism of Action
I. Drug intercalates with DNA by forming hydrogen bonds with the DNA. It then causes cross-linkages in the DNA and thus, causes strand breaks.
II. It interferes with topoisomerase II, which results in prevention of the repairing and recoiling of the damaged DNA strands.[2]
Structural Activity Relationship
- Induction of apoptosis in cell is dependent on the chain length of dimethylaminoalkyl.
- Presence of short side chains can reduce the cytotoxic effects of the drug.
- Th presence of terminal dimethyl groups in the alkylamino side chains are important for the antitumor action of the drug.
- The length of different amino alkyl side chains can result in the different stabilities of the drug-DNA complexes.
- Hydrogen bonding and water bridges can have significant contribution in the intercalation type of molecular complexation.
- Binding enthalpy is mainly responsible for the complexation.
- Melting temperature and free energy changes increases with deacrease in the number of methylene groups in the side chain.
- The maximum biological activity of the drug was found when the number of methylene groups were 2. [3]
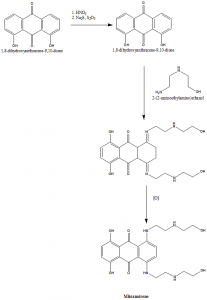
Methods of Synthesis
i. 1,8-dihydroxyanthracene-9,10-dione undergoes nitration and then treated with sodium sulfide to give 1,8-dihydroxyanthracene-9,10-dione.
ii. The above compound formed is treated with 2-(2-aminoethylamino)ethanol followed by oxidation to give mitoxantron.
Therapeutic Uses
Mitoxantrone is used for the treatment of:
- Non-Hodgkin’s lymphomas
- Breast cancers
- Acute myelogenous leukemia
- Advanced prostate cancer
Side Effects
- Common side effects includes low blood counts, fever, increase in blood tests measuring liver functions, nausea and vomiting.
- Some people may suffer from side effects like weakness, low blood pressure, arrhythmias, diarrhea, loss of hair, mouth sores and discoloration of eyes and urine.
MCQs
Q.1 Which term is NOT associated with the drug Mitoxantron?
a) Novantron
b) Blenoxan
c) 1,4-dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]-anthracene-9,10-dione.
d) Mitozantron
Q.2 Match the following with respect to the physical forms of drugs
| i. Mitoxantron | A. Blue-Black solid form |
| ii. Daunorubicin | B. Thin red needle form |
| iii. Dacarbazine | C. Pale yellow crystalline powder form |
| iv. Mettronidazole | D. Ivory microcrystalline solid |
a) i-A, ii-D, iii-C, iv-B
b) i-D, ii-A, iii-B, iv-C
c) i-A, ii-D, iii-B, iv-C
d) i-A, ii-B, iii-D, iv-C
Q.3 The drug Mitoxantron shows its action through
a) Topoisomerase II inhibition
b) Cross-linkgae formation between DNA strands
c) Inhibiting the purine formation
d) Both a) and b)
Q.4 The correct order for the synthesis of the drug Mitoxantrone is
I. Treatment with sodium sulfide
II. 1,8-dihydroxyanthracene-9,10-dione undergoes nitration
III. Oxidation
IV. Reaction with 2-(2-aminoethylamino)ethanol
a) II – III – IV – I
b) I – II – IV – III
c) II – I – IV – III
d) II – IV – III – I
Q.5 Predict the incorrect statements from the following with respect to the classification of the drug.
I. Prednisolone belongs to glucocorticoids.
II. Fulvestrant is selective estrogen reseptor modulator.
III. Fosfestrol is an antibiotic cytotoxic drug.
IV. Mitoxantron is an antibiotic cytotoxic drug.
a) II & III only
b) All of the statements are incorrect.
c) I, II & III only
d) II, III & IV only
Q.6 The correct sequence of True and False for the given statements with respect to the side effects of drug Mitoxantron is?
I. Low blood counts is common side effect of drug.
II. Arrhythmia is not a side effect of the drug.
III. Hair loss may occur in the patient taking the drug.
IV. Decrease in blood tests measuring liver functions.
a) FTFT
b) TTFF
c) TFTF
d) FFTT
Q.7 Which amongst the following drugs is having highest number of ring system in its structure-
a) Mitoxantron
b) Daunorubicin
c) Dacarbazine
d) Metronidazole
ANSWERS
1-b
2-d
3-d
4-c
5-a
6-c
7-b
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Fox EJ. Mechanism of action of mitoxantrone. Neurology. 2004 Dec 28;63(12 suppl 6):S15-8. [3] Harker WG, Slade DL, Drake FH, Parr RL. Mitoxantrone resistance in HL-60 leukemia cells: reduced nuclear topoisomerase II catalytic activity and drug-induced DNA cleavage in association with reduced expression of the topoisomerase II. beta. isoform. Biochemistry. 1991 Oct 1;30(41):9953-61.