FULVESTRANT Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
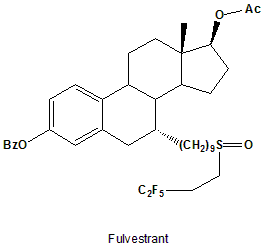
Fulvestrant
IUPAC nomenclature
(7R,8R,9S,13S,14S,17S)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol.
Classification
Fulvestrant is a selective estrogen receptor down regulator. [1]
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 606.8 g/mol |
| 2 | Appearance | Present as white powder form. The solution for injection appears as colorless to yellow viscous liquid. |
| 3 | Melting point | 100-110 °C |
| 4 | Solubility | 9.53X10-6 mg/ L in water |
| 5 | Octanol/water partition coefficient | 9.09 |
| 6 | Presence of ring | Phenanthrene |
| 7 | Number of chiral centers | .6 |
Mechanism of Action
Fulvestrant binds with the estrogen receptors and downregulate them. This leads to the unavailability for the estrogen to get bind to the receptors. Further, fulvestrant degrades the receptors to which they bind. This helps in achieving the anti-estrogen effect which can inhibit the estrogen-sensitive human breast cancer cell lines.[2]
Structural Activity Relationship
- The long side chain at position 7α bends by 90 degrees at its fifth carbon, hugging the surface of the LBD and interacting with the coactivator-binding groove.
- The terminal hydrophobic n-butyl group of ICI 164,384 fits into a pocket formed by the side chains of Leu261, Met264, Ile265 and Leu286 in the coactivator-binding groove of rat ERβ.
- Pure antiestrogenicity is optimal with side chain lengths of 15–19 atoms in a reporter assay in HepG2 cells transiently transfected with ERα.
- The addition of shorter side chains (13 or 14 carbons side chains) results either in agonist or SERM activity.
- Pure antiestrogenicity is associated with chain lengths long enough to reach the coactivator-binding groove.
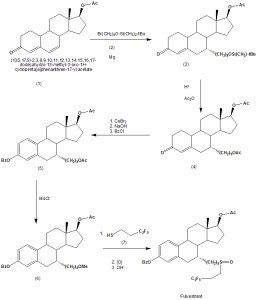
Method of synthesis
i. (1) is reacted with (2) in presence of magnesium metal to give compound (3).
ii. (3) undergoes reduction and reacts with Ac2O to give compound (4).
iii. (4) is then reacted with CuBr2, then with NaOH and then with BzCl to give compound (5).
iv. Compound (5) reacts with MsCl to give compound (6).
v. Compound (6) finally gives the drug fulvestrant by first reacting with compound (7) and then by undergoing oxidation.
Therapeutic Uses
The drug used for the treatment of:
- Estrogen receptor positive metastatic breast cancer in postmenopausal women.
Side Effects
Less common side effects are abdominal pain, diarrhea, constipation, bone pain, headache, sore throat, hot flashes, weakness, cough, injection site reaction, nausea and vomiting.
MCQs
Q.1 ‘Faslodex’ is the trade name of which drug?
a) Finasteride
b) Fulvestrant
c) Fosfestrol
d) Vincristine
Q.2 The addition of shorter side chains (13 or 14 carbons side chains) in the structure of Fulvestrant results in?
a) Agonist activity
b) SERM activity
c) Both a) and b)
d) None of the above
Q.3 Which amongst the following are the correct side effects of the drug?
I. Nausea and vomitting
II. Abdominal pain
III. Hot flashes
IV. Constipation
a) I, II & IV
b) I & II
c) I, II, III & IV
d) I & III
Q.4 Melting point of Fulvestrant is?
a) 100-110 °C
b) 150-160°C
c) 50-60°C
d) 200-210°C
Q.5 Which pairs of drug and its classification are true?
| I. | Prednisolone | Estrogen |
| II. | Fosfestrol | Glucocorticoids |
| III. | Fulvestrant | Selective estrogen receptor down regulators |
| IV. | Toremifene | Selective estrogen receptor modulator |
a) I & III
b) II & IV
c) III & IV
d) I & IV
Q.6 The solution for injection of Fulvestrant appears as?
a) Colourless to yellowish viscous liquid
b) Yellowish non-viscous liquid
c) Reddish-brown viscous liquid
d) Does not available in liquid preparation for injections
Q.7 Type of ring present in Fulvestrant is?
a) Phenanthrene
b) Pteridene
c) Oxazophosphorine
d) No ring structure is present
ANSWERS
1-b
2-c
3-c
4-a
5-c
6-a
7-a
REFERENCES
[1] Tripathi KD. Essentials of Medical Pharmacology, 6thEdn. Jaypee Brothers Medical Publishers (P) Ltd. 2008: 820. [2] Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. British journal of cancer. 2004 Mar;90(1):S2-6.