Gene-Therapy: Concept, Gene Delivery Systems, and Applications and MCQ for GPAT, GATE & CSIR NET
Introduction
The primary goal of Gene therapy is to treat inherited or genetic basics of the diseases. The basic principle of gene therapy involves the manipulation of genes and replacing the defective or non-functional gene which is primarily responsible for the cause of the disease, with the desired gene to cure the diseases.
Concept of Gene therapy
Strategies of gene therapy
- Gene augmentation therapy: In this therapy genetic material is modified by DNA or required gene, to replace the missing gene responsible for a desired gene product
- Gene inhibition therapy: In this therapy expression of dominant gene is inhibited by insertion of Anti-sense gene.
Approaches for Gene Therapy
- Somatic cell gene therapy: In this therapy, the target is to correct the genetic defects in non-reproductive cells like bone marrow cells, blood cells, skin cells, intestinal cells (except sperm cells or egg cells). Somatic cells are altered by insertion of expressible genes to correct genetic basics of disease permanently. The genetic alterations of somatic cells do not pass through successive generations. Hence it is widely preferred.
- Germ cell gene therapy: In this therapy, the target is to correct the genetic defects in reproductive or germ cells. Germ cells are altered by insertion of expressible genes. For ethical, technical, and safety reasons germ cell gene therapy is not practiced at present, because the genetic alterations of germ cells pass to successive generations.
Approaches of Gene therapy based on a procedure
- Ex vivo gene therapy: In this technique gene defect tissue cells are isolated from the patient. The cells are grown by cell culture techniques in laboratories with minimal alteration in environment. Expressible therapeutic gene is replaced into cultured cells to correct defect genes. Then again genetically modified cells are isolated and then cultured. Later, gene-modified cells are isolated and replaced or transformed into the patient.
- In vivo gene therapy: This technique involves delivery of therapeutic genes directly into the cells of particular tissue by viral or non-viral vectors. Hence it is known as in-vivo gene therapy. The potential candidate for in-vivo gene therapy is, tissue cells of skin, liver, spleen, muscle, lung, blood cells, and brain.
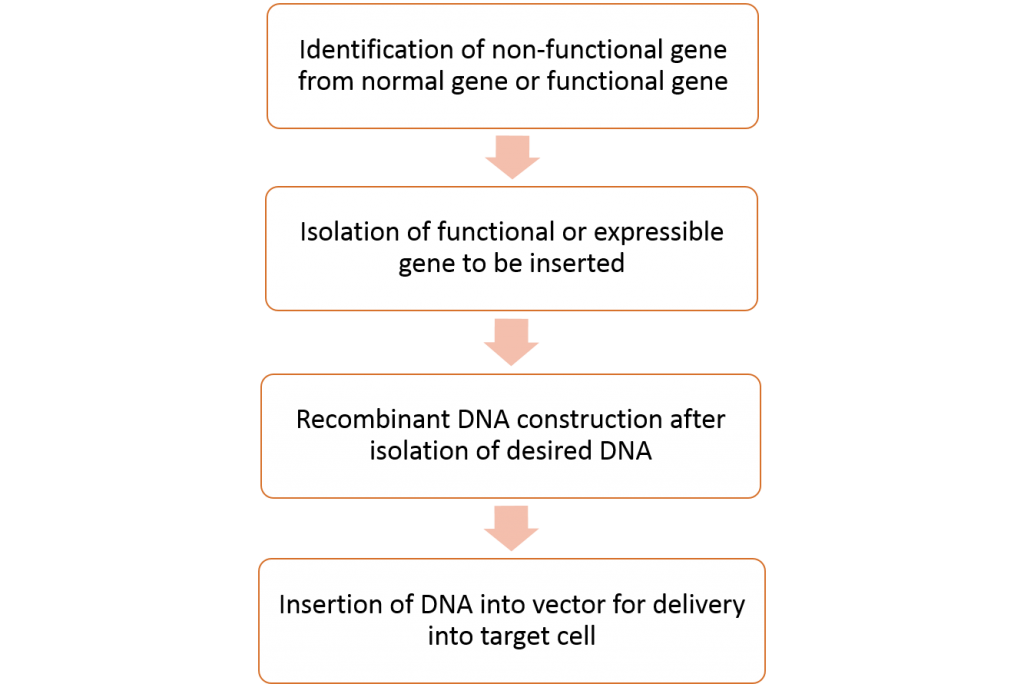
General Procedure for gene therapy
Drug delivery systems or vectors for gene therapy
Several gene delivery systems are used for gene therapy, the carrier molecules used to deliver therapeutic genes to target cells are known as vectors.
Vectors for somatic and Ex-vivo gene therapy
Viruses: Generally retrovirus is used as vectors for ex-vivo gene therapy. As they have RNA genetic material, it synthesis DNA from RNA by reverse transcription hence, viral DNA gets incorporated into a host cell.
Human artificial chromosome (HAC): A synthetic chromosome that can replicate with other chromosomes and can encode a specific desired protein.
Bone marrow cells: Totipotent embryonic stem cells in bone marrow can be used for gene therapy, this totipotent cells can be divided or differentiated into cells like RBC’s, platelets, macrophages, osteoclasts, B- and T-lymphocytes. Bone marrow transplantation is most widely used technique for Ex vivo gene therapy also e.g. sickle cell anemia, SCID, thalassemia.
Vectors for In-vivo gene therapy
Viral vectors: Various virus-like adenovirus, retroviruses, Adeno-associated Viruses, HIV, herpes simplex virus, hepatitis-B virus, human papillomavirus, and Epstein Barr virus are used as vectors in In-Vivo gene therapy. Viral encoded proteins play’s a crucial role in delivery and mechanism of genes.
Non-viral medicated gene delivery systems
- Liposome mediated delivery
- Synthetic retrotransposon vectors
- Oligonucleotide carrier systems
- Self-organized DNA-photonic nanostructures
- Polymer-based gene delivery systems
- Peptide-based gene delivery systems
- Electronic pulse delivery system
- Electroporation
- Aquasomes as gene carriers
- Proton sponge
Applications of Gene therapy
Treatment for type-I diabetes mellitus: A complete sequence of rat pre-pro insulin 1cDNA is encoded in retroviral vector and is delivered into rat liver hepatocytes in-vivo. The results shows a change in glucose homeostasis.
Treatment for hemophilia B: Hemophilia is due to deficiency of coagulation factor IX production which results in severe bleeding. Viral vectors, encoding canine factor IX cDNA is introduced into portal vasculature of hemophilia B dogs. Results shows an increase in factor IX in blood plasma.
Treatment for cardiovascular diseases: Treatment of several CVS diseases can be done by gene modification for specific aliment. Atherosclerosis (gene: HDL), transplant rejection (gene: leukocyte adhesion molecule), aortic aneurysms (gene: Protease) congenital heart diseases (gene: myocytes differentiation factors), myocardial infarction (gene: fibroblast growth factor transforming), can be done by gene augmentation, by using viral vectors or non-viral vectors like liposomes.
Treatment for Alzheimer’s disease: Loss of cognition in Alzheimer’s is due to loss of cholinergic neurons in CNS. Nerve growth factor (NGF) is delivered to prevent neuronal loss. Gene therapy involves intra-cerebral delivery of fibroblasts containing, NGF. Results show a small amount of NGF is produced by cholinergic neuron.
Treatment for cancer: HLA-B7 encoded gene is delivered by liposomes by direct injection in tumor cells. The tumor cell, phagocytize liposomes and express foreign HLA-B7 antigen on their cells. Antibodies recognize these antigens and tumor cells are phagocytized by scavenger cells.
Treatment for AIDS: Gene therapy involves, making HIV resistant cells or inhibition of HIV virus replication. This can be accomplished by use of, Antisense RNA (mRNA) for HIV regulatory protein responsible for replication i.e. ‘tat’ and ‘rev’ mRNAs gene or to reverse transcriptase ‘pol’ and env mRNAs gene is used to suppress replication of HIV-1 retro-Virus
Treatment for orthopedic ailments: Bone, cartilage, tendons, & ligaments are a target site for treatment. The gene expression for growth factors and cytokines is achieved by inserting an expressible gene to a target site by in-vivo or Ex-vivo technique. This approach shows bone development and regeneration. Hence, it is used for rheumatoid arthritis and ligament damage.
Multiple choice questions
- The process in which functional genes are inserted to correct disorders is:
a) Vaccination
b) Stem cell therapy
c) Beta-cell regeneration therapy
d) Gene therapy
2. In gene therapy, functional gene is inserted into target cell should:
a) Replace non-functional gene
b) Make desired amount of protein
c) Stop complete expression of gene
d) Suppress non-expressible gene
3. In gene augmentation therapy genetic material is ______
a) Modified
b) Replaced
c) Suppressed
d) Removed
4. Somatic cell gene therapy is used for following types of cell Except:
a) Bone marrow cells
b) Blood cells
c) Skin cells
d) Sperm cells
5. Germ cell therapy if used for ____________
a) RBC
b) Stomach cells
c) Egg cells
d) Bone marrow cells
6. When gene therapy is done in somatic cells it is ___
a) Not-Heritable
b) Heritable
c) Rarely heritable
d) Not related to heritability
7. Gene therapy can be effective target for _________
a) Hypersensitivity reactions
b) Cancer
c) Diabetes insipidus
d) Type-II diabetes mellitus
8. The commonly used vector in gene therapy to carry target gene into host cells is_________
a) Bacteria
b) Fungi
c) Virus
d) Eukaryotic cell
9. The following are the vectors for somatic gene therapy, Except___
a) Synthetic chromosome
b) Virus
c) Bone marrow cells
d) Liposomes
10. Gene therapy for Alzheimer’s diseases is aimed to _______
a) Deliver nerve growth factor
b) Deliver tumor necrotic factor
c) Deliver Acetylcholine
d) Deliver dopamine
- D
- B
- A
- D
- C
- A
- B
- C
- D
- A
Reference
- S.P. Vyas, V.K. Dixit, Pharmaceutical biotechnology, New Delhi, CBS publishers and distributors, 2008, Page: 401-436
- U. Satyanarayana, U. Chakrapani, Biochemistry, 4th e.d. New Delhi, Elsevier publications, 2013, page: 625-633