GLYCOPYROLLATE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
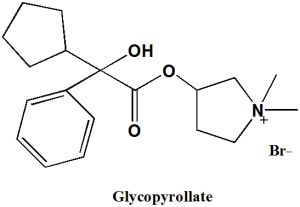
Glycopyrollate
IUPAC nomenclature
(1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetate.
Classification
Glycopyrollate is an acetylcholine antagonist. It is a muscarinic antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 398.3 g/mol |
| 2 | Physical appearance | White crystalline powder |
| 3 | Melting point | 194°C. |
| 4 | Solubility | Soluble in water and alcohol |
| 5 | Presence of ring | Pyrolidine, cyclopentyl and benzene |
| 6 | Number of chiral centers | 3 |
Mechanism of Action
i. The drug binds with muscarinic acetylcholine receptors and inhibits the action of acetylcholine on structure innervated by postganglionic cholinergic nerves and on smooth muscles that responds to acetylcholine.
ii. Exocrine glands, atrioventricular node, sinoatrial node, cardiac muscles and the smooth muscles’s effectors cells are affected due to this.
iii. Free acidity of the gastric secretions decrease and excessive pharyngeal, tracheal and bronchial secretions are also controlled. [1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potet derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
Method of synthesis
i. 2-cyclopentyl-2-hydroxy-2-phenylacetic acid reacts with SOCl2 in methanol to give methyl 2-cyclopentyl-2-hydroxy-2-phenylacetate.
ii. The latter compound reacts with 1-methylpyrollidin-3-ol in presence of sodium metal to give 1-methylpyrrolidin-3-yl 2-cyclopentyl-2-hydroxy-2-phenylacetate.
iii. The latter compound treated with methylbromide in acetone to give a mixture of diastereomers of glycopyrollate.
iv. On crystallization, glycopyrollate in a purified form can be obtained.
Therapeutic Uses
Glycoyrollate is used for:
- Reducing excessive drooling caused by medical conditions.
Side Effects
Side effects of Glycopyrollate are:
- Dry mouth
- Dizziness
- Stomach upset
- Constipation
- Drowsiness
- Allergic reactions
MCQ
Q.1 Maximum potency obtained of glycopyrollate when the distance between the ring substituted carbons is?
a) 1 carbon unit
b) 2 carbon unit
c) 3 carbon unit
d) 4 carbon unit
Q.2 Correct sequence for the True/False for correct IUPAC names of the drug can be?
- Bethanchol: 2-(Carbamoyloxy)-N,N,N-trimethylpropan-1-aminium.
- Glycopyrollate: (–)-(S)-3-Hydroxy-2-phenylpropionic acid (1R,2R,4S,5S,7α,9S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]non-7-yl ester
- Scopolamine: (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetate
- Propranolol: (RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol.
a) FTTF
b) FFFT
c) TFFT
d) TTTT
Q.3 Physical appearance of glycopyrollate is?
a) White crystalline powder
b) Viscous liquid form
c) Red crystals
d) Granular appearance
Q.4 Glycopyrollate produces effect on?
- a) Nicotinic receptors
- b) Muscarinic receptors
- c) Both nicotinic and muscarinic receptors
- d) None of the above
Q.5 Which amongst the following is a therapeutic use of drug glycopyrollate?
a) Treatment of peptic ulcers
b) Treatment of Blurred vision
c) Treatment of excessive drooling
d) None of the above
Q.6 Which of the following drug and their classification are correct?
I. Bethanchol: Cholinergic agonist
II. Glycopyrollate: Acetylcholine antagonist
III. Scopolamine: Nicotinic receptors antagonist
IV. Propranolol: α-adrenergic antagonist
a) I , III
b) I , IV
c) III
d) I, II
Q.7 Steps for synthesis of glycopyrollate from ‘2-cyclopentyl-2-hydroxy-2-phenylacetic acid’?
I. Reaction with SOCl2 in methanol
II. Reaction with methylbromide in acetone
III. Reaction with 1-methylpyrollidin-3-ol
IV. Crystallization
a) I – III – II – IV
b) I – II – III – IV
c) III – I – II – IV
d) II – I – III – IV
ANSWERS
1-b
2-c
3-a
4-b
5-c
6-d
7-a
REFERENCES
[1] Salem MG, Ahearn RS. The effects of atropine and glycopyrrolate on intra‐ocular pressure in anasthetised elderly patients. Anaesthesia. 1984 Aug;39(8):809-12.