HOMATROPIN (HYOSCINE) Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
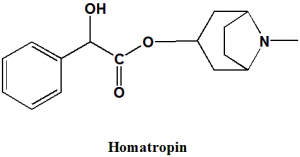
Homatropin
IUPAC nomenclature
(N-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 2-hydroxy-2-phenylacetate.
Classification
Homatropin is a acetylcholine antagonist-muscarinic antagonist.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 275.34 g/mol |
| 2 | Physical appearance | White to fine-white crystalline powder |
| 3 | Melting point | 99.5°C. |
| 4 | Solubility | Water solubility is 5.57 mg/ml |
| 5 | Presence of ring | Benzene and aza bicyclooctane rings present |
| 6 | Number of chiral centers | 4 |
Mechanism of Action
- Homatropin blocks muscarinic receptors and cholinergic signaling pathways.
- It blocks the responses from iris sphincture muscle and cause pupil to become unresponsive to light upon dilation or mydriasis.
- Homatropin also blocks the accommodation muscle of the ciliary body to cholinergic stimulation.[1]
Structure Activity Relationship
- Either R1 or R2 must be heterocyclic or carbocyclic.
- The R3 group can be hydrogen, hydroxyl, hydroxymethyl or amide.
- Most potent derivatives has X as an ester.
- X can also be either oxygen or absent completely.
- The N substituent can be quaternary ammonium salt or tertiary amine or both with different alkyl groups.
- Maximum potency obtained when the distance between the ring substituted carbons is 2 carbon units.
Method of synthesis
i. Tropin and 2-phenyl-2-(vinyloxy)acetyl chloride undergoes esterification reaction to give 8-methyl-8aza-bicyclo[.2.1]octan-3-yl 2-phenyl-2-(vinyloxy)acetate.
ii. The latter compound undergoes acidic hydrolysis to give Homatropin.
Therapeutic Uses
Homatropin is used for:
- Before eye examination
- After eye surgeries
- Treatment of certain eye conditions
Side Effects
Side effects of homatropin are:
- Eye irritation
- Redness of eye
- Burning in eye
- Stinging in eye
- Blurred vision
MCQ
Q.1 Correct statements related with the Physicochemical properties of Homacycline are?
I. Molecular weight = 496 gm/mol
II. Physical appearance : present in viscous liquid form
III. Melting point = 99.5°C
IV. Solubility: very soluble in water
a) I, III
b) I, II, IV
c) II, IV
d) I
Q.2 Match the following of the drugs with their correct IUPAC names.
| i. Methacholine | A. 2-(Acetyloxy)-N,N,N-trimethylpropan-1-aminium. |
| ii. Homatropin | B. (RS)-(8-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate |
| iii. Procyclidine | C. (N-Methyl-8-azabicyclo[3.2.1]oct-3-yl) 2-hydroxy-2-phenylacetate |
| iv. Atropin | D. 1-cyclohexyl-1-phenyl-3-pyrrolidin-1-ylpropan-1-ol |
a) i-B, ii-A, iii-D, iv-C
b) i-A, ii-C, iii-D, iv-B
c) i-D, ii-C, iii-B, iv-A
d) i-A, ii-B, iii-C, iv-D
Q.3 Correct steps for the mechanism of action of the drug Homatropin?
I. Antagonizing muscarinic receptors
II. Agonizing muscarinic receptors
III. Blocks the responses from iris sphincture muscle and cause pupil to become unresponsive to light upon dilation or mydriasis.
a) I – III
b) II – III
c) III – I
d) III – II
Q.4 Correct sequence for True/false for the classification of the drug can be?
- Methacoline: Cholinergic antagonist
- Procyclidine: Muscarinic antagonist
- Homatropin: Cholinergic agonist
- Atropin: Muscarinic antagonist
a) FTFT
b) TTTF
c) TFTF
d) FFTT
Q.5 Maximum potency obtained when the distance between the ring substituted carbons is?
a) 1 carbon unit
b) 4 carbon unit
c) 2 carbon unit
d) 7 carbon unit
Q.6 The correct sequence for the steps for synthesis of drug Homatropin from 2-phenyl-2-(vinyloxy)acetyl chloride?
I. Reaction with trimethylamine
II. Reaction with acetic anhydride
III. Esterification with tropin
IV. Hydrolysis
a) I – II
b) I – III – II
c) I – III – II – IV
d) III – IV
Q.7 Side effect of drug Homatropin?
a) Burning in eye
b) Redness of eye
c) Blurred vision
d) All of the above
ANSWERS
1-a
2-b
3-a
4-a
5-c
6-d
7-d
REFERENCES
[1] Christensen RE, Pearce I. Homatropine hydrobromide: Effect of topical administration upon the intraocular pressure and aqueous facility values of normal and chronic simple glaucomatous eyes. Archives of Ophthalmology. 1963 Sep 1;70(3):376-80.