HYDROXYAMPHETAMINE Synthesis, SAR, MCQ,Structure,Chemical Properties and Therapeutic Uses
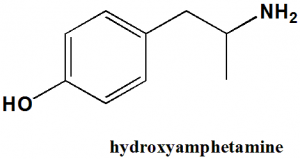
Hydroxyamphetamine
IUPAC nomenclature
4-(2-aminopropyl)phenol
Classification
Hydroxyamphetamine is an indirect acting sympathomimetic amine drug having adrenergic properties.
Physiochemical Properties
| S. NO. | PHYSICAL AND CHEMICAL PROPERTIES | |
| 1 | Molecular weight | 151.21 g/mol |
| 2 | Physical appearance | White to off-white crystalline powder |
| 3 | Melting point | 125.5°C |
| 4 | Solubility | Water solubility is 3.14 mg/ml |
| 5 | Presence of ring | Benzene ring |
| 6 | Number of chiral centers | 1 |
Mechanism of Action
i. Hydroxyamphetamine causes release of norepinephrine from adrenergic nerve.
ii. This results in mydriasis. [1]
Structure Activity Relationship
The SAR of Adrenergic agonist can be discussed as follows:
- Primary or secondary aliphatic amine separated by two carbons from a substituted benzene ring is essential for the high agonist activity.
- The hydroxyl substituted carbon must be in the R configuration for the maximal direct activity.
R1 substitution:
- When R1 is increased in size, activity of alpha receptors decreases and activity of the beta receptors increases
- Activity of both alpha and beta receptors is maximum when R1 is methyl group.
- Alpha agonist activity decreases when R1 is larger than methyl, and went negligible when R1 is isopropyl.
- Large lipophillic groups can afford compounds with alpha blocking activity.
- N-substituent provides selectivity for different receptors.
- Arylalkyl group can provide beta selectivity, increased cell penetration and increased lipophillicity for the longer duration of action.
R2 substitution:
- Ethyl group can eliminate the alpha activity of the drug.
- Erythrostero isomers have maximal activity.
- The additional methyl group makes the drug more selective for the alpha2
R3 substitution on the aromatic ring:
- 3’,4’-dihydroxy substituted benzene ring has poor oral activity.
- 3’, 5’-dihydroxy compounds are orally active.
- At least one of the groups is required which can form hydrogen bonds. And if only one group is present then it is preferred at 4’ position to retain the beta2
If phenyl group has no phenolic substituent then it may act directly or indirectly. [2]
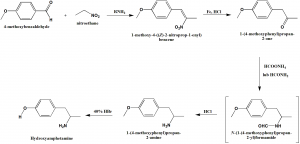
Method of synthesis
i. 4-methoxybenzaldehyde and nitroethane reacts together in presence of primary amine to give 1-methoxy-4-((Z)-2-nitroprop-1-enyl)benzene.
ii. The latter compound in presence of iron and HCl gives 1-(4-methoxyphenyl)propan-2-one.
iii. On reaction with an amide, an unstable compound is produces which on treatment with HCl produces 1-(4-methoxyphenyl)propan-2-amine.
iv. On reaction with 40% HBr, hydroxyamphetamine is produced.
Therapeutic Uses
Hydroxyamphetamine is used:
- for widening of the of the pupil during any inflammatory conditions.
- For the diagnosis of Horner’s syndrome.
Side Effects
Side effects of hydroxyamphetamine are:
- Allergic reactions
- Irregular heart beats
- Hypertension
- Redness and tearing in eyes
- Nervousness
- Tremors
- Headache
- Blurred vision
- Dowsiness
- Dizziness
- Sweating
- Nausea
MCQs
Q.1 ”4-(2-aminopropyl)phenol” is the IUPAC nomenclature of which drug?
a) Hydroxyamphetamine
b) Ephedrine
c) Metaraminol
d) Propylhexedrine
Q.2 Physical appearance of Hydroxyamphetamine is?
a) Liqiud at room temperature
b) White to off-white crystalline powder
c) Red crystalline powder
d) Yelllow oily liquid at room temperature
Q.3 Match the following with correct classifications of the drugs.
| i. Hydroxyamphetamine | A. Camptothecin analogue |
| ii.Methotrexate | B. Folate antagonist antimetabolite |
| iii. Phenoxybenzamine | C. Indirect acting sympathomimetic |
| iv. Topotecan | D. Nonselective α-adrenergic antagonist |
a) i-B, ii-A, iii-D, iv-C
b) i-C, ii-A, iii-D, iv-B
c) i-D, ii-A, iiiB-, iv-C
d) i-C, ii-B, iii-D, iv-A
Q.4 Hydroxyamphetamine causes release of………… which results in ………..?
a) epinephrine, mydriasis
b) norepinephrine, mydriasis
c) acetylcholine, reduced blood pressure
d) acetycholine, increase in blood pressure
Q.5 Correct sequence for True and False for the given statements related with the SAR of drug…..
- Primary or secondary aliphatic amine separated by two carbons from a substituted benzene ring is essential for the high agonist activity.
- The hydroxyl substituted carbon must be in the S configuration for the maximal direct activity.
a) TF
b) FT
c) TT
d) FF
Q.6 Number of chiral centers present in the structure of Hydroxyamphetamine?
a) 0
b) 1
c) 2
d)3
Q.7 The drug Hydroxyamphetamine is mainly used for the diagnosis of?
a) Tumors
b) Kidney stone
c) Homer’s syndrome
d) None of the above
ANSWERS
1-a
2-b
3-d
4-b
5-a
6-b
7-a
REFERENCES
[1] Gill JR, Mason DH, Bertter FC. Studies of the Natriuresis of Hydroxyamphetamine and on Its Mechanism of Action. Annals of Internal Medicine. 1966 May 1;64(5):1185. [2] Lemke TL, Zito SW, Roche VF, Williams DA. Essentials of Foye’s principles of medicinal chemistry. Wolters Kluwer; 2017, 348-352